Lung cancer is the leading cause of cancer death in Australia, accounting for 8466 deaths in 2015, and 5.3% of deaths overall. It causes more deaths than breast or prostate cancer, although it is diagnosed less frequently.1 The five-year survival rate for lung cancer remains extremely low at 15.8%,2 which is attributable to the delayed emergence of symptoms and resulting late stage at diagnosis.3
Nonetheless, there have been unparalleled advances in the management of advanced lung cancer enabling meaningful prolongation of survival. These include targeted therapies directed at actionable somatic tumour mutations as well as immune checkpoint inhibitors that reactivate the host’s immune response against the cancer. Additionally, prevention and early diagnosis coupled with curative treatments are key strategies for reducing lung cancer mortality by avoiding the sequelae of metastatic disease.
This brief update discusses early lung cancer, concentrating on diagnosis, which is key for enabling successful curative treatments. These now include video-assisted thoracoscopic surgery, conventional and stereotactic ablative radiotherapy, as well as combined chemoradiation/adjuvant immunotherapy for locally advanced disease. Therefore, the main focus of the article is on priority advances in screening and early diagnosis that are likely to translate to practice.
Screening
Some cancers, such as breast cancer, have much better outcomes than lung cancer. This is at least in part due to early diagnosis or early-stage curable disease, contributed to by an effective national screening program. Although screening by chest X-rays has been definitively shown in randomised controlled trials (RCTs) not to reduce lung cancer mortality,4 the advent of high-quality low-dose computed tomography (LDCT) screening is amassing increasing evidence for potential change to practice through policy.
In 2011, the National Lung Cancer Screening Trial was published, comparing annual chest X-rays to LDCT for lung cancer screening. The study investigated 53,454 people at high risk for lung cancer with annual LDCT over three years. ‘High risk’ was defined by the following criteria: age 55–74 years, ≥30 pack-year smoking history and being a current or ex-smoker within the past 15 years. Results showed a 20% relative reduction in mortality from lung cancer deaths with LDCT. The rate of positive LDCT results was 24.2%, with 96.4% ultimately being false positives. Of patients with a positive LDCT result, 58.9% went on to have some form of clinical procedure. Complications post–diagnostic procedure occurred at a rate of 1.4% and were more likely to occur if lung cancer was present (11.2% , compared with 0.06%). The average effective dose of LDCT was 1.5 mSV, compared with 8 mSv for a diagnostic computed tomography (CT) scan of the chest.5 Subsequently, lung cancer screening has been recommended by the United States Preventive Services Task Force in their 2014 recommendation statement and implemented in the USA.6
Confirmation of the effectiveness of CT screening came from the recent NELSON RCT conference report of reduced lung cancer deaths at 10 years by 26% in men and an even larger percentage in women.7 The 2019 MILD trial also suggested a persistent benefit in reduction of mortality with prolonged screening beyond five years.8
In Australia, it has been shown that that high-quality LDCT screening is feasible and that long-term smoking cessation in a CT screening population is effective; the costs of screening and associated impacts on quality of life have also been reported.9–11 The International Lung Screen Trial is currently underway within Australia to assess the benefits of risk prediction models for patient selection to improve the efficiency of CT screening. A 2013 Australian survey of 90 patients at risk of lung cancer found the majority would be willing to participate in screening with LDCT.12
Lung cancer screening is not currently recommended by the Australian Standing Committee on Screening; however, with mounting evidence, Federal Health Minister Hunt has called for an inquiry.13 Discussion is expected to note the integral role of smoking cessation in any screening program, and the unique potential of CT screening (unlike screening for other cancers) to detect important and treatable diseases including ischaemic coronary artery calcification, emphysema and osteoporosis.
Pathways to diagnose suspected lung cancers
The two main principles of lung cancer diagnosis are tissue diagnosis and staging. Tissue biopsy is required to confirm lung cancer diagnosis, determine the subtype (eg small cell, squamous cell, adenocarcinoma) and provide material for further testing for targetable tumour mutations (such as EGFR, ALK, ROS-1) and programmed death-ligand 1 (PD-L1) expression. Staging is commonly accomplished using a CT scan and positron emission tomography/CT scan, with biopsy of thoracic lymph nodes or sites of distant metastasis to confirm tumour involvement if needed. Accurate diagnosis and staging are prerequisites for identifying early curable disease and guide treatment options for advanced lung cancer. This diagnostic approach is illustrated in Figure 1. Early curable lung cancers are typically small nodules with limited nodal involvement. Methods of biopsy for these nodules include bronchoscopy and transthoracic needle aspiration (TTNA). Developments in classic fibreoptic bronchoscopy including navigation bronchoscopy and endobronchial ultrasonography (EBUS) to biopsy peripheral lesions have changed practice, enabling diagnostic biopsies in people for whom the alternative procedure of TTNA may be contraindicated. While modestly more accurate, TTNA biopsy has higher complication rates than bronchoscopy; major complication rates (pneumothorax requiring intervention, haemothorax, air embolism, needle tract seeding, and death) are approximately 5%, compared with <1% for EBUS.14–18
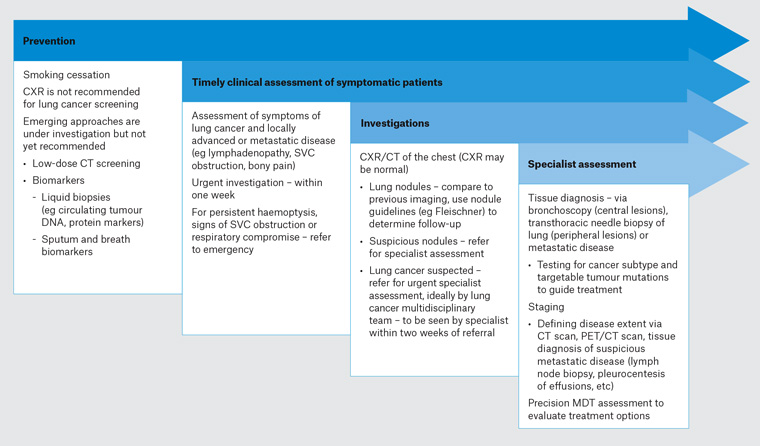
Figure 1. An approach to lung cancer diagnosis in 202035. Click here to enlarge
CT, computed tomography; CXR, chest X-ray; MDT, multidisciplinary team; PET, positron emission tomography; SVC, superior vena cava
Managing lung nodules
As a result of the increasing use of CT technology, a greater number of nodules are encountered in clinical practice. Most of these will be benign, but the challenge is determining how best to manage these nodules in order to diagnose early lung cancers without subjecting those with benign nodules to unnecessary investigations, each with potential harms and benefits. Although there are no specific Australian guidelines, a number of high-quality guidelines are readily available and commonly used; The Fleischner Society,19 British Thoracic Society,20 American College of Chest Physicians21 and Cancer Council Australia (www.cancer.org.au/health-professionals/clinical-guidelines/lung-cancer.html) have produced evidence–based nodule and cancer guidelines (Table 1). The guidelines are important tools that can reduce healthcare variation and patient harm, and improve patient outcomes and communication. The guidelines suggest risk assessment of nodule and patient (eg nodule size, location; patient age, smoking history, cancer history, etc) and review of historical CT scans to ascertain stability. One emerging challenge for the adoption of these guidelines in Australia is the increasing reliance on volumetrics for threshold assessment and for sensitive identification of short-term growth as a marker for malignant potential, which is not routine clinical practice.
The implication of these technological advances is that it is now much easier and safer to accurately diagnose and stage patients presenting with suspected lung cancer, thus avoiding the risks of empirical treatment of suspected but pathologically unconfirmed lung cancer. However, they do not have a first-line role in the screening of asymptomatic individuals, which has led to efforts to develop non-invasive tests for lung cancer.
| Table 1. Relevant Australian and international guidelines on pulmonary nodule surveillance and pathways for lung cancer diagnosis that may be of use for Australian general practitioners |
| Australian guidelines |
| Cancer Council Australia |
Guidelines outlining optimal care for lung cancer by stage – including stepwise practice advice on timelines, investigations and management. Two-page summary available.
|
| Cancer Australia |
A two-page practical summary to guide general practitioners on risk factors and investigating symptoms of lung cancer.
|
| Summaries of the optimal framework for quality lung cancer care for professionals and consumers.
|
| International guidelines |
| Fleischner Society |
Guidelines on incidentally detected pulmonary nodule follow up. Includes photographic demonstration of nodule characteristics.
|
| British Thoracic Society |
Guidelines on initial and follow-up approach for pulmonary nodules as well as detailed evidence-based recommendations for diagnosis and management of lung cancer updated in March 2019.
|
| American College of Chest Physicians (ACCP) |
Detailed summary of pulmonary nodule follow-up and diagnosis and management of lung cancer. Includes table of investigation harm and benefits.
|
Biomarkers
There has been a surge of interest in lung cancer biomarkers in recent years as researchers evaluate biological specimens such as sputum, saliva, buccal swabs, breath condensate and breath volatile organic compounds (VOCs). Thousands of resulting publications describe these potential lung cancer biomarker candidates. These minimally invasive tests are relatively safe, acceptable to consumers and can be repeated more frequently than tissue biopsy, which for some people with lung cancer is not feasible or too high risk because of comorbidities.
Breath biomarkers
Analysis of exhaled breath for VOCs is a new approach to the diagnosis of lung cancer. As early as Roman times, the smell of a person’s breath has assisted physicians with the diagnosis of a disease; for example, uncontrolled diabetes was associated with a sweet, acetone odour;22 liver failure produced a fish-like smell,23 and renal failure was identified by a urine-like smell.24 McCulloch showed that dogs could be trained to detect lung cancer and breast cancer in people with various stages of disease with almost 100% accuracy, merely by smelling the subject’s breath.25 These observations suggest that there are biomarkers in exhaled breath that are potentially useful for diagnosing disease. Studies examining exhaled breath using gas chromatography and mass spectrometers have identified individual chemical compounds associated with lung cancer and confirmed that there is not one single VOC, but rather a combination of VOCs.26 However, these techniques have limited applicability in the clinical setting because of their expense and difficulty of use, and the need for highly experienced analysts to operate and interpret the results. Electronic noses and related instruments are simpler, cheaper and easier to use, facilitating their utilisation in the clinical setting. These instruments employ different sensor array technologies to identify VOC patterns or smellprints rather than individual compounds. Studies using a variety of these instruments, different sampling techniques and different statistical analyses have consistently discriminated between groups of subjects with lung cancer and control subjects.27 Small studies have shown a sensitivity of the exhaled breath VOC test between 73% and 100%, and specificity between 48% and 100%. Therefore, further research needs to be performed before it can become a clinical tool. Large-scale multicentre clinical trials and validation in independent at-risk subjects are required to improve the clinical sensitivity and specificity of the test. 28
Blood biomarkers
Liquid biopsy aims to detect and quantify circulating cancer biomarkers by analysing biological fluids, predominantly blood samples. These biomarkers contain abundant genomic, proteomic and metabolomic information that can be used for early disease detection (diagnostic biomarkers) and to predict treatment response and relapse (prognostic/predictive biomarkers). Biomarkers include tumour-associated antigens, tumour-associated autoantibodies, circulating tumour cells, circulating tumour DNA (ctDNA), microRNA and exosomes.
Early lung cancer with low-volume disease has intrinsic challenges for platforms that require demonstration of direct tumour attributes (such as somatic tumour DNA mutations), as the low amounts of ctDNA in blood in early lung cancer relative to late-stage cancer are below the diagnostic limit of detection (even with the current next-generation sequencing platforms). Some biomarkers may be more effective when used in combination with traditional serum cancer markers such as the multi-analyte CancerSEEK (ctDNA and protein) test. Although some biomarkers have progressed to RCTs in clinical populations,29–32 none has achieved sufficient levels of analytical/clinical validity and clinical utility to enter mainstream use.
Key implications
Anti-cancer therapeutics are dramatically changing outcomes of metastatic lung cancer. They are benefitting a subset of responders but are counterbalanced by a different spectrum of treatment toxicities including financial ones. Earlier in the natural history, prevention and screening for curable disease can help avoid metastatic disease. Lung cancer screening with CT provides a 20% or greater reduction in lung cancer mortality. It has already been implemented overseas and is currently under investigation in Australia. Nodules are a very common incidental finding that should be managed in line with international and/or national guidelines; it is important that healthcare providers familiarise themselves with these to avoid over-investigation and under-diagnosis of cancer. In the meantime, important national resources are available to assist with achieving best practice and outcomes for people at risk of and with lung cancers. Namely, use of the Australian Optimal Care Pathways, with demonstrated ability to prolong survival in colorectal cancer, and the Cancer Australia lung cancer guidance and the Lung Cancer Framework.33,34 Both emphasise timely assessment and referral of suspected lung cancer for tissue diagnosis, staging and consideration of patient preferences and comorbidities by a multidisciplinary team to achieve optimal outcomes. An active partnership between primary care physicians and their local multidisciplinary teams is key to enabling cure where possible and palliation to optimise quality of life otherwise for all affected by lung cancer.