Case
A woman aged 55 years presented to her general practitioner (GP) with a painful, rapidly progressing ulcer surrounding her ileostoma site. The lesion initially appeared one week prior as a ‘small pimple’. Physical examination revealed an ulcer measuring 8 cm × 5 cm. It had violaceous and undermined borders (Figure 1).
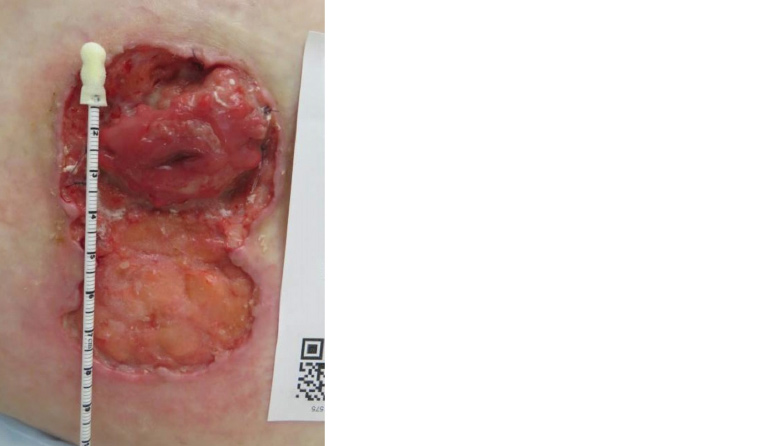
Figure 1. Peristomal ulcer measuring approximately 8 cm × 5 cm with violaceous undermined borders
The patient had a resection of her sigmoid colon, right hemicolectomy and end-ileostomy in 2015 for segmental colitis associated with perforated diverticular disease. In 2018, she developed colitis of the diverted colon, which was conservatively managed. She was classified as obese (class 3) because of a body mass index of 48 kg/m2. Her other medical history included osteoarthritis, non-alcoholic fatty liver disease, fibromyalgia and allergic rhinitis. Her medications included mesalazine (rectal), duloxetine, psyllium husk capsules, fexofenadine and oxycodone. She had medication allergies to sulfonamides and celecoxib.
The GP referred the patient to the emergency department for further assessment and investigation, and she was subsequently admitted under the care of the gastroenterology team. Laboratory tests were unremarkable, apart from a mildly elevated C-reactive protein level (14 mg/L; reference range <6 mg/L). A punch biopsy of the ulcer edge showed a deep dermal neutrophilic dermatosis. Tissue culture and stains were negative for infective microorganisms.
Question 1
What is the most likely diagnosis?
Question 2
What differential diagnoses should be considered?
Answer 1
Although uncommon, the most likely diagnosis is pyoderma gangrenosum. It is one of a group of autoinflammatory diseases known as neutrophilic dermatoses. The Maverakis criteria1 published in 2018 are 86% sensitive and 90% specific if the single major criterion is met (histopathology of ulcer edge shows a neutrophilic infiltrate) along with ≥4 minor criteria, which include:
- exclusion of infection
- pathergy (the development of cutaneous lesions at sites of trauma)
- history of inflammatory bowel disease (IBD) or inflammatory arthritis
- history of papule, vesicle or pustule ulcerating within four days
- peripheral erythema, undermining border, tenderness at the ulcer site
- multiple ulcers, with at least one on the anterior lower leg
- cribriform or wrinkled paper scars at the site of the healed ulcer
- decreased size of the ulcer within one month of initiating immunosuppressive medication.1
Answer 2
Infective aetiology should be ruled out, including atypical mycobacterium, botryomycosis, necrotising fasciitis or deep fungal infection. A malignant ulcer – namely a basal cell or squamous cell carcinoma, including Marjolin type – must be excluded. It is also important to consider a venous, arterial or neuropathic ulcer; traumatic or pressure ulcer; Sweet’s syndrome; cutaneous Crohn’s disease; calciphylaxis; cholesterol embolism; small- or medium-vessel vasculitis; vasculopathy; warfarin-induced skin necrosis; antiphospholipid antibody syndrome; medication reaction or spider bite.
A diagnosis of pyoderma gangrenosum can only be made once other diagnostic possibilities have been excluded through clinical, histopathological and laboratory findings.
Case continued
For this patient, the results of extensive serological testing – which included a full blood examination, chemistry profile, hepatitis screen, serum and urine protein electrophoresis, vasculitis screen and hypercoagulability studies – were unremarkable. Thus, laboratory and biopsy findings enabled exclusion of other diagnoses such as malignancy, infection, vasculitis and thrombotic disease. Furthermore, the patient fulfilled the diagnostic criteria for pyoderma gangrenosum as suggested by Maverakis et al. Therefore, a diagnosis of pyoderma gangrenosum was madesignificant improvement, and all glucocorticoid-related side effects had resolved (Figure 2).
Question 3
What are the risk factors for developing pyoderma gangrenosum?
Answer 3
Pyoderma gangrenosum can occur at any age but is most common between the ages of 40 and 60 years.2,3 More than half of all patients with pyoderma gangrenosum have underlying systemic disease, most commonly IBD, inflammatory arthritis and haematological disease.3 Peristomal pyoderma gangrenosum is uncommon, typically occurs in patients with IBD and is likely induced by irritation from stoma secretions or trauma to the skin caused by stoma maintenance.2
Case continued
The patient was discharged from hospital with an outpatient dermatology appointment in four weeks’ time. However, the ulcer rapidly progressed in the interim, leading the patient to re-present to her GP, who then initiated systemic treatment. The patient was prescribed 50 mg of daily oral prednisolone and referred urgently for dermatology review. Treatment was continued with adjunctive topical and intralesional glucocorticoids as well as wound and stoma cares. After four weeks, there was no clinical improvement and the patient had developed significant side effects including glucocorticoid-induced diabetes mellitus and oral candidiasis. Glucocorticoid-sparing treatment options were discussed, and adalimumab (40 mg per week) was commenced. A six-week weaning regimen for prednisolone was established concurrently. After eight weeks, the peristomal ulcer showed significant improvement, and all glucocorticoid-related side effects had resolved (Figure 2).
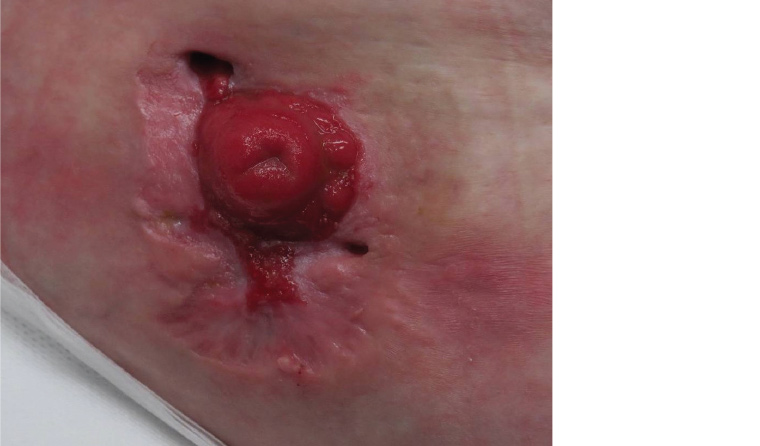
Figure 2. Follow-up after eight weeks of adalimumab therapy. The peristomal ulcer has re-epithelialised, with significant reduction in size.
Question 4
What are the options for systemic treatment of more extensive or rapidly progressing pyoderma gangrenosum ulcers?
Answer 4
When systemic treatment is needed, glucocorticoids are the first-line treatment and can produce a rapid response.4 Prednisolone used at a dose of 0.5–1.5 mg/kg per day (maximum daily dose of 60 mg) is most common. Tapering and/or discontinuation should occur within 4–10 weeks to prevent glucocorticoid-related side effects. Response to therapy is varied, and the ulcers often take months to heal.5 Therefore, glucocorticoid-sparing agents are often required, such as dapsone, cyclosporine, tumour necrosis factor–α inhibitors, mycophenolate mofetil, methotrexate or azathioprine.
In this patient, an appropriate trial of systemic glucocorticoids failed to achieve the desired clinical outcome to warrant continuation of this treatment. Furthermore, the development of glucocorticoid-induced diabetes mellitus precluded the use of cyclosporine, a common second-line agent used in the treatment of pyoderma gangrenosum. Dapsone was contraindicated because of the patient’s history of sulfonamide allergy. Other standard systemic therapies were avoided because of their potential for significant toxicities and unpredictable outcomes.6 Given the severity of her disease, adalimumab was proposed as a second-line therapy because of its favourable side-effect profile when compared with other glucocorticoid-sparing agents, ease of administration and demonstrated efficacy in a growing number of pyoderma gangrenosum cases published in recent years.6–8
Recognition of the pathergy phenomenon in pyoderma gangrenosum is important for optimal management of these patients. All non-urgent elective surgical or other traumatic procedures should be avoided during the active stage of pyoderma gangrenosum because of the risk of additional pathergy at these sites.
Key points
- New validated diagnostic criteria for pyoderma gangrenosum were published in 2018.
- Peristomal pyoderma gangrenosum is uncommon and often difficult to manage.
- Systemic glucocorticoids have a role in the management of pyoderma gangrenosum.
- If there is limited clinical response to systemic glucocorticoids within the first 1–2 weeks of use, it is important to consider urgent referral to dermatology for consideration of adjunctive therapy.
- Careful monitoring and specialist involvement are vital when using systemic agents for pyoderma gangrenosum, as treatment can often be prolonged.