Diabetic Charcot foot (DCF) is a progressive degenerative arthropathy, and its formation is postulated to originate from repeated microtrauma due to the loss of protective sensation as a result of diabetic sensory neuropathy.1,2 The presentation of DCF can be acute or chronic. Acute DCF is characterised by unilateral foot swelling with erythema, elevated foot temperature and a bounding peripheral pulse. This is often misdiagnosed as osteomyelitis and cellulitis, especially in the presence of foot ulcers and absence of foot deformities and X-ray abnormalities. Distinguishing between infection and DCF in the acute stage is challenging, especially when availability of investigations is limited in the primary care setting. When a patient with diabetes presents with soft tissue and bone deformity of the foot in addition to loss of protective sensation, a high index of suspicion of DCF should be made to initiate appropriate management.3
DCF may cause gross structural deformities of the foot and ankle and subsequent skin ulceration leading to lower limb amputation if it is not managed early with appropriate measures. Several studies have observed multiple complications after DCF for a follow-up period ranging from two to 12 years.4–10 Foot ulcers have been reported as one of the most common complications after DCF in most observational studies, occurring in 11–60% of cases.5,6,8,10 The need for surgery and amputation at the DCF site was also observed during follow-up.4,5,7–10 Between four and five years of DCF follow-up, the mortality rate was observed to be 21–33%.5–7 Of 115 patients followed up by Fabrin et al, 43% had developed recurrent acute Charcot foot within the median of two years of follow-up.8 In another study of 301 patients with DCF, 28% were noted to have impaired mobility as one of their complications over a follow-up period of 2.5 years.9
Foot ulcers, lower limb amputation, foot surgery and mortality had been observed in previous studies as complications following DCF. There is still a paucity of literature on DCF complications and its related associated factors in Asia, hence the primary objective of this study. The four main complications explored were recurrent foot ulcers, foot surgery, amputation and mortality.
Methods
This was a retrospective cohort study. Data were obtained from patients attending the Diabetic Foot and Wound Management Clinic of University Malaya Medical Centre (UMMC) from 2001 to 2014 through the clinic attendance census. Patients without diabetes who presented with Charcot foot were excluded from the study. The study was approved by the UMMC Ethics Committee (MECID:201401-0659).
A face-to-face interview was conducted to gather details on patients’ sociodemographic statuses. Physical examination was performed to determine each patient’s current foot status, followed by a review of the patient’s medical records to determine management received. For deceased subjects, only their medical record data were reviewed.
Ninety-eight sets of data for patients with DCF were collected between 2001 and 2014, with a mean follow-up time of 5.11 years. From the total data, 83 patients (84.7%) were still alive during the study period. The four main complications identified were foot ulcers, foot surgery, amputation and mortality. Inferential statistical analysis was performed using simple and multiple logistic regressions (SPSS version 21.0) to determine associated factors for all DCF complications. Kaplan–Meier survival analysis was generated to obtain the mean survival time for mortality.
Results
Descriptive results from the demographic profile showed equal sex distribution, with 48.2% male patients (n = 40) and 51.8% female patients (n = 43). The mean age of the patients was 59.2 ± 9.2 years. Table 1 describes the details of demographic profiles.
In terms of medical comorbidities, 86.7% (n = 72) had hypertension, 71.1% (n = 59) had dyslipidaemia, 39.8% (n = 33) had ischaemic heart disease and only 21.7% (n = 18) had end-stage renal failure. In addition, 89.1% (n = 68) had diabetes for >10 years and 76% (n = 63) had poorly controlled glycated haemoglobin (HbA1c) with readings of >48 mmol/mol (6.5%).
Acute and chronic Charcot foot were diagnosed by the attending physician at the Diabetic Foot and Wound Management Clinic, and diagnoses were based on physical examination with radiological investigation (foot X-ray). Table 2 summarises the DCF profile descriptions.
| Table 1. Demographic profiles (n = 83) |
| Demographic |
Frequency (n) |
Percentage |
| Sex |
Male |
40 |
48.2 |
| Female |
43 |
51.8 |
| Age (years) |
<50 |
12 |
14.5 |
| 51–70 |
64 |
77.1 |
| >70 |
7 |
8.4 |
| Ethnicity |
Malay |
44 |
53.0 |
| Chinese |
7 |
8.4 |
| Indian |
32 |
38.6 |
| Education level |
Secondary school |
47 |
56.6 |
| Tertiary education |
18 |
21.6 |
| Occupation |
Homemaker/Unemployed |
16 |
19.3 |
| Desk job |
17 |
20.5 |
| Non–desk job |
50 |
60.2 |
| Table 2. Diabetic Charcot foot profiles (n = 83) |
| Diabetic Charcot foot information |
Frequency (n) |
Percentage |
Diabetic Charcot foot
during diagnosis |
Acute |
54 |
65.1 |
| Chronic |
29 |
34.9 |
| Current diabetic Charcot foot status |
Acute |
5 |
6.0 |
| Chronic |
75 |
90.4 |
| Amputated |
3 |
3.6 |
| Ulcer during diagnosis |
Present |
42 |
50.6 |
| Current ulcer |
Present |
38 |
45.8 |
| Non-surgical treatment |
Yes |
72 |
86.7 |
| No |
11 |
13.3 |
| Removable boot walker |
Yes |
23 |
31.9 |
| Modified shoes |
Yes |
62 |
86.1 |
| Casting |
Yes |
33 |
45.8 |
| Padding |
Yes |
10 |
13.9 |
| Foot insole |
Yes |
69 |
95.8 |
With regard to the main complications in patients with DCF, 75.9% (n = 63) had recurrent ulcers followed by foot surgery and lower-limb amputation (Table 3). Other secondary outcomes were ambulation and driving status after being diagnosed with DCF. Findings showed that 53.0% (n = 44) required aid for ambulation and only 32.5% (n = 27) continued to drive.
| Table 3. Complications of diabetic Charcot foot during follow-up* (n = 83) |
| Complications |
Frequency (n) |
Percentage |
| Recurrent ulcer |
Present |
63 |
75.9 |
| Absent |
20 |
24.1 |
| Surgical treatment |
Yes |
44 |
53.0 |
| No |
39 |
47.0 |
| Arthrodesis |
Yes |
44 |
100.0 |
| Exostomy |
Yes |
7 |
15.9 |
| Wound debridement |
Yes |
38 |
86.4 |
| Incision and drainage |
Yes |
18 |
40.9 |
| Amputation status |
Yes |
38 |
45.8 |
| Yes |
45 |
54.2 |
| Amputation level |
Rays |
28 |
73.7 |
| Transmetatarsal |
1 |
2.6 |
| Transtibial |
9 |
23.7 |
| *Mean follow-up time of 5.11 years |
The majority of patients (n = 63; 75.9%) had recurrent ulcers post-diagnosis of DCF, and 44.4% (n = 28) had more than three episodes of recurrent ulcers. Analysis with confounder adjustments showed that patients with ulcers and DCF had a higher risk of recurrent ulcers (odds ratio [OR] 4.27; 95% confidence interval [CI]: 1.38, 13.21; P = 0.01) than those without ulcers (Table 4).
A total of 44 patients (53%) had foot surgery after DCF. The majority of foot surgeries occurred during the initial year of diagnosis (n = 26; 59.1%). Multiple logistic regression analysis showed that patients with recurrent foot ulcers had a higher risk of foot surgery (OR 3.41; 95% CI: 1.13, 10.32; P = 0.03) than those without recurrent ulcers when adjusted for other confounding variables (Table 4).
Almost half of the patients with DCF (n = 38; 45.8%) had complications with lower-limb amputation. Most of the amputations (n = 20; 52.6%) occurred during the first two years following diagnosis of DCF. Multiple logistic regression analysis showed that patients with recurrent ulcers had eight times the likelihood of having an amputation (OR 8.47; 95% CI: 1.83, 39.16; P = 0.006) followed by heart disease (OR 5.23; 95% CI: 1.42, 19.20; P = 0.01) and chronic DCF (OR 3.91; 95% CI: 1.12, 12.99: P = 0.03; Table 4).
| Table 4. Multiple logistic regression analysis on predictors of Charcot foot complications |
| Outcomes |
Associated factors |
Odds ratio |
95% confidence interval |
P value |
| Lower limit |
Upper limit |
| Recurrent ulcer |
Ulcer during diagnosis |
Present |
4.27 |
1.38 |
13.21 |
0.012 |
| Foot surgery |
Recurrent ulcers |
Present |
3.41 |
1.13 |
10.31 |
0.030 |
| Amputation |
Ischaemic heart disease |
Yes |
5.23 |
1.42 |
19.20 |
0.013 |
| Stage of Charcot at diagnosis |
Chronic |
3.91 |
1.12 |
12.99 |
0.026 |
| Recurrent ulcers |
Present |
8.47 |
1.83 |
39.16 |
0.006 |
The mortality outcome during the follow-up period was 15.3% (n = 15). The majority of the 15 deceased patients were between 51 and 70 years of age (n = 10) and had macrovascular and microvascular complications of diabetes. The mean survival time based on Kaplan–Maier survival analysis was 44.8 ± 5.5 months (Figure 1).
In relation to mobility outcomes, almost 88% of the patients (n = 73) were ambulating without aid prior to being diagnosed with DCF. Patients with foot ulcers at diagnosis had a significant association with aided premorbid ambulation (P = 0.039) in comparison to patients without ulcers at diagnosis.
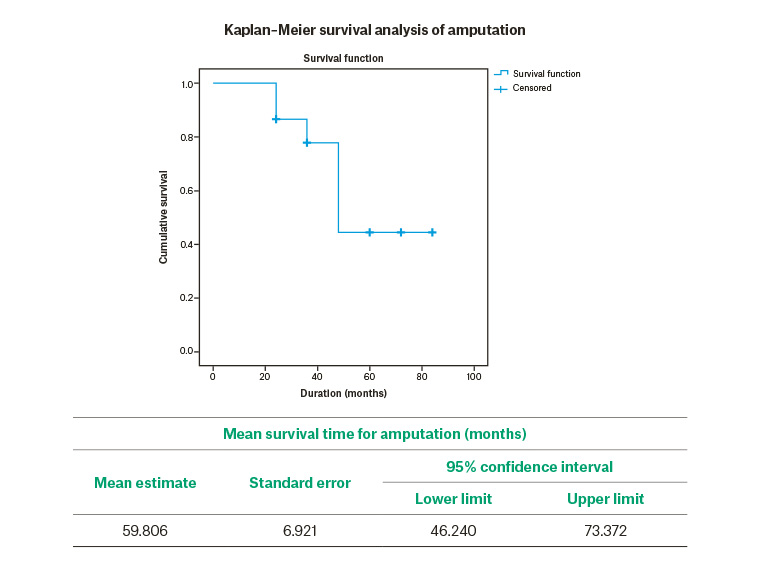
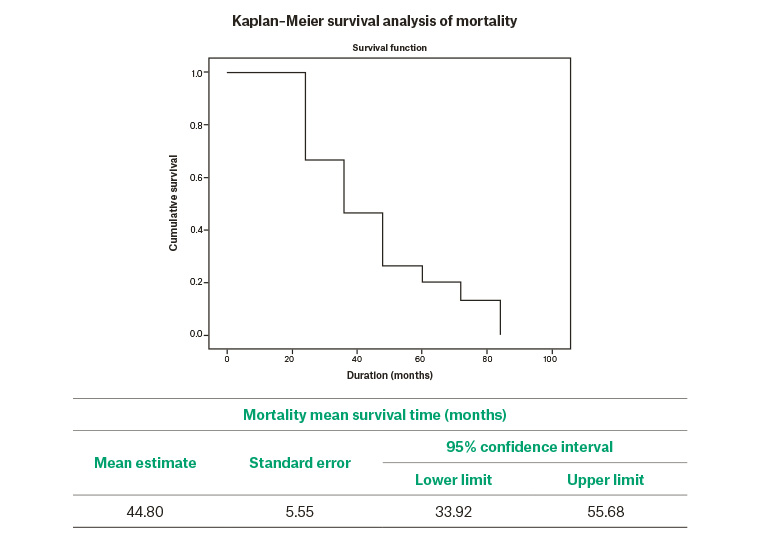
Figure 1. Kaplan–Meier survival analysis graphs for amputation and mortality
Discussion
Complications related to DCF were the result of a complex cascade of diabetes and neuropathy causing distortion of the normal bony architecture of the foot, which can result in the development of rocker bottom foot.2,11 The deformity and ankle instability cause ulceration and infection, which subsequently lead to the requirement for foot surgery and amputation.12
Recurrent ulcers after DCF were more frequent in the current study (75%) when compared with previous studies (11–67%).6,8,13 Patients who presented with an ulcer with DCF were four times more likely to have recurrent foot ulcers later. Foot surgery was performed for 53% of the patients, which is similar to the percentage reported in other studies.1,6,8 Furthermore, other studies noticed a bimodal distribution for surgical intervention during the first year and third to fourth year post-diagnosis due to recurrent ulcers, foot infections and foot deformities.1,6,8 Patients with DCF who had recurrent ulcers were more likely to undergo foot surgery (OR 3.413).
Of the patients who had surgery, more than 50% had wound debridement and incision and drainage related to infective ulcer management. Corrective surgery with arthrodesis was performed in all patients who underwent surgery. Arthrodesis and exostomy are commonly done for unstable and deformed Charcot foot.1 A timely surgery, adequate strong fixation and a long non–weight bearing postoperative period of at least 12–18 weeks are necessary to enhance good outcomes in DCF.13 Surgical intervention with reconstruction surgery also needs to be carefully evaluated and justified.1,14 Findings of surgical outcomes after DCF indicate that almost half of the patients with DCF presented with unstable joint and foot deformity. In this case, ongoing weight-bearing may lead to uneven pressure distribution at the plantar of the foot, hence creating ulceration and leading to infection that warrants management (eg with debridement and incisional drainage).15
This study also showed a higher percentage of lower limb amputation (45.8%) than other similar studies, which reported figures of between 4.1% and 23.4%.4,5,8 Patients with recurrent ulcers had a higher risk of having an amputation than patients without recurrent ulcers.16 The overall amputation risk for Charcot foot was found to be not significantly different from diabetic foot ulcers.4 Nevertheless, the observations in this study showed that the presence of Charcot foot deformity with ulcers predicts a higher risk of lower limb amputation.
Uncontrolled HbA1c levels were seen in 75.9% (n = 63) of patients in this study. These findings are similar to another study that reported that <15% of patients had HbA1c <58 mmol/mol (7.5%). Samann et al also concluded that HbA1c was not a predictive factor for frequent complications of DCF.17
A combination of diabetic foot ulcers and the need for surgery has previously been identified as the main predictor of developing DCF in the same population of people with diabetes at the UMMC.18 This factor had also been observed to be a strong predictor of DCF complications independent of other factors in this study. The presence of ulcers with DCF was significantly associated with amputation risk, foot surgery, development of recurrent ulcers and reduction of ambulation capability.
Although most patients in the current study received non-surgical management (86.7%), which constitutes pressure offloading devices, the occurrence of foot ulcers remained high. Some studies have found that non-adherence to pressure offloading devices has no predisposing effect in the development of ulcers and foot surgery in DCF, which can be due to multiple other confounding factors.8,13 However, in acute DCF, usage of and adherence to pressure offloading devices reduced the risk of further complications related to foot ulceration.19 In a multi-ethnic community in Malaysia, the cultural norm is for indoor ambulation without any shoes for the majority of ethnicities, which can be a possible factor in poor pressure offloading of the DCF. Uneven plantar foot pressure distribution predisposes patients with DCF to foot ulceration. Adequate offloading and plantar distribution management are among some of the main strategies to prevent debilitating complications of diabetic foot problems.14 Assessment of compliance with the usage of pressure offloading devices prescribed may give better insight into treatment effectiveness to reduce DCF complications.
The mortality outcome during the follow-up period was 15.3% (n = 15). Other studies have reported mortality percentages from 3.63% to 44.7%.5–7,9,16,20 The main cause of death in the current study was medical complications of diabetes. This result is similar to the study by Moulik et al, which indicated that clinical conditions related to cardiovascular origin, such as cardiac arrest and stroke, contributed to the majority of causes of death of patients with DCF.16
There are several limitations of this study, especially in obtaining data on the type of treatment done and foot complication status for deceased subjects because of missing data in the medical record. In this study, patients’ compliance for non-surgical intervention was not assessed because of its retrospective nature, which may lead to recall bias. Nevertheless, comparison of outcomes between patients on total contact casting and with removable pressure offloading devices might be useful in future prospective studies where missing data and recall bias can be avoided. Another limitation relates to peripheral vascular disease, which is one of the common comorbidities among patients with diabetes who have foot concerns but was not captured during data recruitment.
Conclusion
Foot ulceration was identified as the main factor associated with DCF complications, which include recurrent ulcers, amputation, foot surgery and declining ambulation status. Prevention of ulceration is of utmost importance in reducing the occurrence of DCF complications. Early recognition at the primary care level and active management of DCF may reduce the risk of developing further complications.
Implications for general practice
- This study focused on DCF, which is a debilitating complication of diabetes.
- Data presented here were from a tertiary care and referral centre in Malaysia.
- Understanding of the complications and predictors of DCF can be applied in clinical services to improve management of DCF and prevent its complications.
- The presence of foot ulcers was the factor most associated with DCF complications.
- Regular assessment of DCF is a core prevention strategy for reducing the risk of DCF complications.