Background
The importance of interconception care – defined as care given to women, and their partners, between one pregnancy and the next to optimise their health – is increasingly important, with rising rates of overweight, obesity, diabetes and hypertension among people of reproductive age. Women frequently visit their general practitioner (GP) in the first six months postpartum. This is an opportune time to discuss ideal interpregnancy intervals (IPIs) and advise women about contraception and healthy behaviours.
The importance of preconception care and optimising one’s health prior to pregnancy is well understood; however, challenges remain in its implementation. Almost half of all pregnancies in Australia are unintended (either an unwanted or mistimed conception),1 and many clinicians do not initiate preconception consultations.2,3 Women and men of reproductive age are increasingly overweight or obese, and have chronic diseases such as diabetes and hypertension.4 Interconception care is defined as the care given to women, and their partners, between one pregnancy and the next. Medicare data show that women visit their general practitioners (GPs) an average of eight times in the first six months postpartum,5 making this an opportune time to discuss optimising health prior to the next pregnancy. The time interval between pregnancies is known to affect the incidence of adverse pregnancy outcomes. Described in various ways (Table 1), the preferred term for the time between pregnancies is ‘interpregnancy interval’ (IPI). Several international organisations recommend an IPI of two years (minimum 18 months) to enable the reduction of risks.6–8 There is no standardised, universal definition of short and long IPIs (Table 1), yet both carry a risk of complications, with more adverse outcomes having been associated with short intervals.9–11
| Table 1. Terms used to describe the time between pregnancies |
| Term |
Definition |
| Interpregnancy interval (IPI) |
The time from the end of one pregnancy to the start of the next pregnancy |
| Birth-to-birth (BTB) interval |
The period between consecutive live births. The BTB interval disregards abortions and fetal deaths, so calculation of the BTB interval can be the same for two women even if one woman conceives only twice and the other conceives multiple times between births. Further, the BTB interval can be the same for two women even if one has a preterm delivery (having conceived later than the other). |
| Short interpregnancy interval |
Less than 18 months from the end of one pregnancy to the start of the next pregnancy has been most commonly used (including by the World Health Organization),8 but recent studies indicate that adverse outcomes are only associated with IPIs less than 12 months21 |
| Long interpregnancy interval |
More than 60 months from the end of one pregnancy to the start of the next pregnancy |
Interconception care involves the active consideration of outcomes of the previous pregnancy or pregnancies in planning for the next one, and it entails education regarding the optimal spacing of pregnancies, the provision of reliable postpartum contraception and consideration of lifestyle risk factors (Figure 1). It also provides an opportunity to tackle maternal health issues that affect pregnancy. These include conditions such as obesity and diabetes, which may have arisen or were perhaps not adequately addressed in the index pregnancy.
Figure 1. Components of the interconception care discussion
This article reviews available research and guidelines on IPIs and interconception care, including controversies regarding the significance of IPIs in obstetric outcomes, and proposes best-practice care for the general practice setting.
Why is the interpregnancy interval important?
The IPI can be regarded as a ‘modifiable’ risk factor because, with the provision of appropriate and effective contraception, women can control when their next pregnancy occurs. Both extremes of IPIs have been linked to an increased risk of poorer outcomes in infants and their mothers.9,10,12
Short interpregnancy intervals
There is a body of literature that has documented the association between short IPIs and adverse events. Theories for the increased incidence of adverse outcomes with short IPIs include the maternal depletion hypothesis, in which there is insufficient time for women to re-establish an optimal nutritional state prior to pregnancy; infectious causes; and deficient recovery of uterine scars.13 Women with shorter IPIs are more likely to experience placental abruption, placenta praevia,14 uterine rupture (for women who previously delivered by caesarean section)14,15 and gestational diabetes.16 Neonatal adverse outcomes include an increased risk of stillbirth, small size for gestational age, preterm delivery and neonatal death.17–19
However, recent studies have suggested the relationship between short IPIs and adverse outcomes may not be as significant as previously thought.20 Such studies have suggested that other associations with short IPIs, such as socioeconomic status, and the risk of starting a subsequent pregnancy while obese may be integral to adverse pregnancy outcomes.16
The authors of the most recent large study examining short IPIs and perinatal outcomes argued that short IPIs do carry an increased risk of adverse outcomes, and the profile of these outcomes changes corresponding to maternal age. They showed that younger women (aged 20–34 years) had higher rates of preterm delivery, and that women aged ≥35 years had higher rates of maternal mortality and morbidity.21
Long interpregnancy intervals
Similarly, long IPIs have historically been associated with an increased risk of pre-eclampsia22 and labour dystocia.9 Theories for the increased risk of adverse outcomes associated with long IPIs include: diminished physiological adaptations to pregnancy over time (eg decreased uterine blood flow) and other factors that contribute to delayed fertility.9 Avoidance of a long IPI is more problematic, since a desired pregnancy may be precluded by factors such as subfertility, availability of a partner, economic or occupational issues, or illness.23
Challenges with implementing the interpregnancy interval
A challenge to implementing the recommended interpregnancy interval is that women frequently do not have a clear understanding of what is the ideal time to wait between pregnancies. Women are often counselled after caesarean section to avoid a rapid conception because of the risk of uterine rupture, but it is uncommon for women to be given advice after a vaginal delivery. In an Australian study of 344 women who had had two or more pregnancies, 20.9% of women had an IPI of <12 months, and only 7.5% of these women believed this was ideal. An IPI of <12 months following a live birth was significantly associated with younger age and with non-use of long-acting reversible contraception (LARC), breastfeeding <12 months and women’s perception that a shorter IPI was ideal. Fewer than half of the women reported having received advice about IPI, and fewer than half about postnatal contraception.24 The study concluded that prevention of short IPIs could be achieved with improved access to postnatal contraception.
Social circumstances must also be considered. Women who are older may feel they do not have the option to delay subsequent pregnancies, balancing age-associated risks of subfertility, miscarriage and chromosomal abnormalities with the advantages of a longer IPI. These are complex conversations that may require a number of consultations to discuss the advantages and disadvantages of delaying conception.
Reducing the frequency of short interpregnancy intervals
The most effective strategy to reduce the frequency of short IPIs is to ensure that women are provided with reliable postpartum contraception. A range of contraceptive options are suitable in the postpartum period, and the medical eligibility criteria for contraceptive use vary only slightly by breastfeeding status (Tables 2 and 3). Of all the methods, LARC – including intrauterine devices (IUDs) and implants – is the most effective, and international studies have shown that the use of LARC can reduce unintended pregnancies when compared with the contraceptive pill.25 Although the use of LARC methods in Australia more than trebled between 2002 and 2013 to 11% of women using contraception,26 access to these methods in the immediate postpartum period remains limited. Improving postpartum uptake may assist women with birth spacing and help avoid short IPIs, and it is supported by many international organisations including the American College of Obstetricians and Gynecologists.27
The provision of immediate postpartum LARC is safe for most women, including those who are breastfeeding, according to the Faculty of Sexual and Reproductive Health.28 Both implants and IUDs can be inserted after caesarean section or vaginal delivery, although the expulsion rate of immediately inserted IUDs is approximately 15–20%.29 Despite the risk of expulsion, the use of IUDs is still worthwhile and cost effective, as many women do not return for their LARC insertion once discharged.29–31
Therefore, discussing the option of immediate postpartum LARC with women is evidence-based. This option should initially be discussed with women at an antenatal consultation. Provision of immediate postpartum LARC may also allow for greater time at the postpartum visit to screen for postpartum anxiety or depression and monitor breastfeeding, as well as address future pregnancy plans and reflect on changes to the mother’s health or risks on the basis of conditions identified during the recent pregnancy.
| Table 2. United Kingdom Medical Eligibility Criteria (UKMEC) for Contraceptive Use28 |
| UKMEC |
Definition of category |
| Category 1 |
A condition for which there is no restriction for the use of the method |
| Category 2 |
A condition where the advantages of using the method generally outweigh the theoretical or proven risks |
| Category 3 |
A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the method is not usually recommended unless other more appropriate methods are not available or not acceptable. |
| Category 4 |
A condition which represents an unacceptable health risk if the method is used |
| Reproduced with permission from The Faculty of Sexual & Reproductive Healthcare (FSRH) |
| Table 3. Summary of United Kingdom Medical Eligibility Criteria for Contraceptive Use categories applicable to women after childbirth28 |
| Condition |
Cu-IUD |
LNG-IUS |
IMP |
DMPA |
POP |
CHC |
| Breastfeeding |
|
| a) 0 to <6 weeks postpartum |
See below |
1 |
2 |
1 |
4 |
| b) >6 weeks to <6 months (primarily breastfeeding) |
1 |
1 |
1 |
2 |
| c) >6 months postpartum |
1 |
1 |
1 |
1 |
| Postpartum (non-breastfeeding women) |
|
| a) 0 to <3 weeks |
|
| (i) With other risk factors for VTE |
See below |
1 |
2 |
1 |
4 |
| (ii) Without other risk factors |
1 |
2 |
1 |
3 |
| b) 3 to <6 weeks |
|
| (i) With other risk factors for VTE |
See below |
1 |
2 |
1 |
3 |
| (ii) Without other risk factors |
1 |
1 |
1 |
2 |
| c) >6 weeks |
1 |
1 |
1 |
1 |
| Postpartum (breastfeeding/non-breastfeeding, including post-caesarean) |
| a) 0 to <48 hours |
1 |
1 |
See above |
| b) 48 hours to <4 weeks |
3 |
3 |
| c) ≥4 weeks |
1 |
1 |
| d) Postpartum sepsis |
4 |
4 |
CHC, combined hormonal contraception; Cu-IUD, copper-bearing intrauterine device; DMPA, progestogen-only injectable: depot medroxyprogesterone acetate; IMP, progestogen-only implant; LNG-IUS, levonorgestrel-releasing intrauterine system; POP, progestogen-only pill; VTE, venous thromboembolism
Reproduced with permission from The Faculty of Sexual & Reproductive Healthcare (FSRH) |
Optimising health before the next pregnancy
Providing access to contraception is vital, but interconception care also involves addressing lifestyle risk factors and maternal and paternal health issues prior to the next pregnancy. Australian women have higher rates of smoking, obesity, diabetes and hypertension with increasing parity (Figure 2), all of which should be considered in the interconception period.32 Contributing factors, such as increasing maternal age and increased cumulative weight gain with each pregnancy, may compound these issues, stressing the importance of good interconception care. The Royal Australian College of General Practitioners’ guidance on preconception care provides a comprehensive list of preventive health behaviours that ought to be raised with a woman and her partner before and between pregnancies (Box 1).33
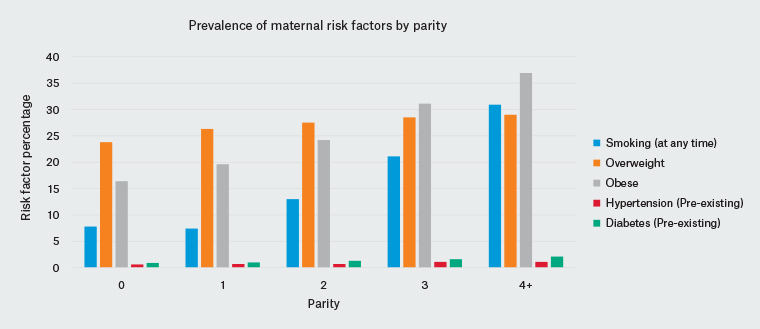
Figure 2. Incidence of lifestyle and medical conditions with increasing parity32
| Box 1. Preconception care checklist33 |
Diet
- Discuss nutritional requirements including folic acid supplementation
- Provide advice about a healthy diet
Weight
- Measure body mass index and provide appropriate advice
Exercise
- Advise 150 minutes of exercise per week or 30 minutes on most days
Pregnancy history
- Screen for any modifiable risk factors
Genetic screening
- If indicated from personal/family history or ethnic background, discuss genetic carrier screening
Smoking/alcohol/illicit drugs
- Assess intake and provide appropriate advice
Psychosocial aspects
- Screen for domestic violence
- Screen for mental health conditions
Medical conditions
- Review current disease status and medications
- Referral/correspondence with specialist if required
Environmental
- Assess work, home and recreational environments
Contraception/family planning
- Offer appropriate contraception advice for those not desiring pregnancy
Breast examination
Dental health check
Screening for sexually transmissible infections and other infectious diseases
- Measles, mumps, rubella, varicella zoster, hepatitis B, syphilis
- Human immunodeficiency virus and hepatitis C with appropriate pre-test counselling
- Cervical screening
|
Given Australia’s rising rate of obesity, body weight is the single most important modifiable risk factor for a range of adverse pregnancy outcomes including prematurity, stillbirth, congenital anomalies and macrosomia, as well longer-term risks of obesity and metabolic disease in children born to obese mothers.34 Unfortunately, meta-analyses of randomised controlled trials show that interventions to limit weight gain during pregnancy have a minimal impact on clinical outcomes;35 therefore, pregnancy may be too late to address the risks of maternal obesity.36 The Royal Australian and New Zealand College of Obstetricians and Gynaecologists statement on obesity in pregnancy proposes that prevention of the short- and long-term effects needs to be achieved through preconception management.
A discussion of weight loss management should be prefaced with an explanation of why a normal body mass index (BMI) range (18.5–24.9 kg/m2) is ideal to optimise fertility and improve pregnancy outcomes, and the impact that even modest weight reduction can have. Several recent publications from large population-based cohorts show the potential benefit of weight loss prior to conception. A Canadian cohort of 225,000 pregnancies found that a 10% decrease in preconception BMI could decrease stillbirth risk by 10%.37 A Swedish birth registry study highlighted the adverse effect of weight gain between pregnancies by showing a dose-response association between interpregnancy weight gain and risk of stillbirth;38 reassuringly, women who were initially overweight but lost weight before a second pregnancy had 50% lower neonatal mortality than women who remained overweight.
The role of the general practitioner in interconception care
Primary care practitioners are uniquely placed to deliver all aspects of interconception care, but research shows they may lack the time and resources to do so.3,39 Support through online tools and easy-to-access checklists, as well as the initiation of discussions about birth spacing and the use of postpartum contraception in antenatal shared-care visits, can facilitate this.3 GPs routinely see parents with their babies for vaccinations or health concerns, and it is recommended that they consider scheduling a specific consultation to discuss interconception care issues. As the prevalence of obesity and chronic diseases increases, interconception care has the potential to reduce future adverse perinatal outcomes. Further research is required to identify how best to target these women at high risk prior to their next conception.
Key points
- Challenges associated with the implementation of preconception care remain. These include high rates of unplanned pregnancies, reduced clinician initiation of preconception consultations, time constraints and lack of supporting resources.
- Interconception care includes a discussion about birth spacing and the optimisation of health for women and their partners prior to the next pregnancy, which is increasingly important in the context of rising rates of chronic disease.
- The recommended IPI is between two and five years to reduce the incidence of adverse pregnancy outcomes, but more recent evidence on short IPIs suggests women should wait at least 12 months after a birth to conceive again.
- Access to reliable methods of postpartum contraception, particularly LARC methods, are the most effective way to achieve the recommended interpregnancy interval.