Chronic kidney disease (CKD; Box 1) is a major health concern in Australia, with a prevalence of 9% among non-Indigenous adult Australians and 18% among Aboriginal and Torres Strait Islander people.1 Risk factors for CKD are listed in Box 2, while Figure 1 shows the classification of stages of CKD by glomerular filtration rate (GFR) and urinary albumin.2 CKD is not itself a diagnosis, and a full assessment includes the three elements shown in Table 1. Attempts should always be made to ascertain the underlying pathology as this helps guide treatment. Recommended diagnostic investigations in CKD are listed in Box 3; these may be varied according to clinical findings and comorbidities (Table 2).3
| Box 1. Definition of chronic kidney disease2 |
- An estimated or measured glomerular filtration rate (GFR) <60 mL/min/1.73m2 that is present for at least three months with or without evidence of kidney damage; or,
- Evidence of kidney damage with or without decreased GFR that is present at least three months, as evidenced by albuminuria, haematuria after exclusion of urological causes, structural abnormalities (eg on kidney imaging tests) or pathological abnormalities (eg on kidney biopsy).
|
| Adapted with permission from Kidney Health Australia, Chronic kidney disease (CKD) management in primary care, 4th edn, Melbourne, Vic: Kidney Health Australia, 2020. |
| Box 2. Risk factors for chronic kidney disease in the Australian population2 |
Potentially modifiable:
- Obesity
- Hypertension
- Diabetes
- Smoking
- Established cardiovascular disease
- History of acute kidney injury
Unmodifiable:
- History of kidney failure in first- or second-degree relative
- Aged 60 years or older
- Aboriginal or Torres Strait Islander
- Maori or Pacific Islander
|
| Box 3. Recommended diagnostic investigations for every patient with chronic kidney disease3 |
- Full blood count, erythrocyte sedimentation rate, C-reactive protein
- Repeat (within one week) serum urea, electrolytes, creatinine, estimated glomerular filtration rate, albumin
- Urine albumin-to-creatinine ratio (first morning void)
- Fasting lipids (including high-density lipoprotein, low-density lipoprotein) and glucose
- Urinalysis and phase-contrast microscopy
- Renal ultrasonography
|
| Table 1. Three-part diagnosis of chronic kidney disease |
| Stage |
Urine albumin-to-creatinine ratio |
Underlying cause |
| 1–5 based on estimated glomerular filtration rate |
Normal
Microalbuminuria
Macroalbuminuria |
Pathology |
| For example, ‘Stage 3 chronic kidney disease with macroalbuminuria due to diabetes’ |
| Table 2. Investigations that may be indicated for patients with chronic kidney disease |
| Patient factors |
Suggested investigations |
| Has or is at risk of diabetes |
Fasting blood glucose, glycated haemoglobin |
| Has eGFR <60 mL/min/1.73m2 |
Serum calcium, phosphate, parathyroid hormone, 25-hydroxy-vitamin D, iron studies |
| Is aged >40 years |
Serum and urine electrophoresis |
| Has rash, arthritis or features of connective tissue disease |
Antinuclear antibodies, extractable nuclear antibodies, anti-dsDNA, complement components (C3, C4) |
| Has pulmonary symptoms or deteriorating kidney function |
Antiglomerular basement membrane antibody, Antineutrophil cytoplasmic antibody |
| Has risk factors for HBV, HCV or HIV |
HBV, HCV, HIV serology |
| Has persistent albumin-to-creatinine ratio >60–120 mg/mmol and/or haematuria (with no urologic cause) |
Consider renal biopsy – refer to nephrologist |
| eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus |
Most people with CKD do not undergo kidney biopsy, so the diagnosis is often presumptive, based on epidemiology, clinical features and investigations including urinalysis and microscopy. Among patients undergoing biopsy in preparation for renal replacement therapy, the most common pathologies are diabetic nephropathy, glomerulonephritis and hypertensive kidney disease.4 Diabetic nephropathy is normally associated with micro- or macroalbuminuria; it is worth noting that not all patients with diabetes and CKD have diabetic nephropathy. The hallmark of glomerulonephritis is haematuria with dysmorphic red cells and casts. The urine is often normal in hypertensive kidney disease, and it may also be normal in other conditions such as polycystic kidney disease and interstitial nephritis.
Although typically asymptomatic in its earlier stages, CKD tends to progress and may result in end-stage kidney disease. CKD is also a risk factor for cardiovascular disease and, in its later stages, is associated with various complications that affect the patient’s wellbeing. Management therefore focuses on delaying progression, reducing cardiovascular risk and preventing or ameliorating complications. Kidney Health Australia, and many guideline bodies internationally, recommend an approach to management based on stage of GFR and albumin excretion (Figure 1).2
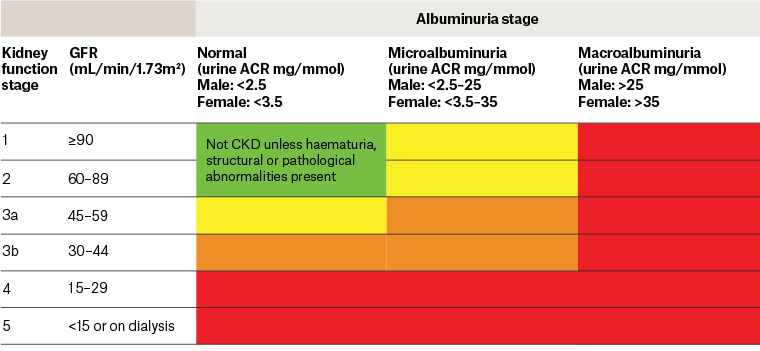
Figure 1. Classification of chronic kidney disease.2 The colours link to recommended management plans in the publication Chronic kidney disease (CKD) management in primary care.2
ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; GFR, glomerular filtration rate
Reproduced with permission from Kidney Health Australia, Chronic kidney disease (CKD) management in primary care, 4th edn, Melbourne, Vic: Kidney Health Australia, 2020.
Slowing progression of chronic kidney disease
Strategies to delay progression of CKD include lifestyle advice, prevention of acute kidney injury, control of blood pressure and albuminuria, and disease-specific interventions. Obesity and smoking are significant risk factors for CKD progression and should be addressed. Physical activity is beneficial, and alcohol consumption should not exceed National Health and Medical Research Council guidelines. With rare exceptions, patients with CKD should be encouraged to maintain a normal protein intake of 0.75–1 g/kg/day. Limiting sodium intake to no more than 100 mmol (6 g salt) per day will benefit both blood pressure and albumin excretion.2,3 Patients should be advised to choose low salt foods and not add salt at the table. Referral to a dietician may be beneficial.
Acute kidney injury is diagnosed by either a sudden increase in serum creatinine or a significant reduction in urine output when compared with normal. Inadvertent use of nephrotoxic medications is a common cause of acute injury; medications that should be avoided or prescribed in reduced dose in patients with CKD include lithium, aminoglycosides, calcineurin inhibitors, gadolinium, radiographic contrast agents, nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors. Trimethoprim can raise serum creatinine by inhibiting tubular secretion – thus reducing estimated GFR (eGFR), which is calculated using serum creatinine – but has no effect on actual GFR.5 A second common reason for acute kidney injury is hypotension, however caused.
Patients with CKD stages 3–5 are at increased risk of acute kidney injury;6 if they are ill and unable to maintain adequate fluid intake (eg due to gastroenteritis), patients should be advised to temporarily cease medications that may increase the risk of decline in kidney function or cause adverse effects as a result of reduced clearance. A useful mnemonic for these drugs is SAD MANS (Sulfonylureas, Angiotensin converting enzyme [ACE] inhibitors, Diuretics, Metformin, Angiotensin receptor blockers [ARBs], Non-steroidal anti-inflammatories, Sodium–glucose co-transporter-2 [SGLT2] inhibitors).
Hypertension is extremely common among patients with CKD because of several interacting mechanisms.7 Control of blood pressure in many cases slows progression of GFR decline and also reduces cardiovascular risk. A significant proportion of patients with CKD have so-called ‘masked hypertension’ with normal blood pressure in the clinic but elevation on ambulatory or home monitoring, so such measurement should be considered. Lifestyle interventions to reduce blood pressure should be discussed with all patients with CKD. Reduction in sodium intake is particularly important, along with weight loss and a low-fat diet rich in fruit and vegetables (eg the Dietary Approaches to Stop Hypertension [DASH] diet).8
Most patients with CKD and hypertension have elevated levels of angiotensin II, which increases systemic vascular resistance and hence blood pressure through direct vasoconstriction. First-line medication for hypertension in CKD is therefore an ACE inhibitor or ARB. These should not be combined. The currently recommended target blood pressure in patients with CKD is consistently less than 130/80 mmHg.2 Aiming for a lower systolic blood pressure of below 120 mmHg may be appropriate in certain individuals at high cardiovascular risk, but the increased risk of falls, syncope, electrolyte abnormalities and acute kidney injury needs to be considered.7
A single agent will not achieve blood pressure control in many patients. Add-on options should be considered in light of comorbidities and may include a thiazide or thiazide-like diuretic, or a calcium channel blocker. Difficulty achieving control may be due to poor medication adherence,9 high sodium intake or obstructive sleep apnoea. If blood pressure is not consistently below target with at least three anti-hypertensive agents, then referral to a nephrologist may be beneficial. Further guidance on blood pressure control in CKD is available in the Kidney Health Australia handbook Chronic kidney disease (CKD) management in primary care.2
Although various proteins may be present in excess in the urine of patients with CKD, testing for albuminuria has better analytical precision and exhibits greater sensitivity than proteinuria testing for detecting most clinically significant abnormalities. Therefore, the recommendation is to use albumin-to-creatinine ratio (ACR) as a standardised measure of protein excretion in most patients with CKD.10 Albuminuria is an independent risk factor for cardiovascular disease, conferring an increased risk at every stage of CKD.11 Reduction in the amount of albuminuria is associated with improved outcomes.12 Strategies include reducing sodium intake and blood pressure, especially through prescription of an ACE inhibitor or ARB. Addition of spironolactone can also reduce albumin excretion; serum potassium should be carefully monitored. Persisting significant albuminuria (eg urine ACR ≥30 mg/mmol) is an indication for referral to a nephrologist.
A number of diseases affecting the kidneys, such as immunological conditions or polycystic disease, need specific treatments that lie outside the scope of this article. However, one recent important discovery is the potential renal and cardiovascular benefits of SGLT2 inhibition in patients with type 2 diabetes. Although originally developed to reduce blood glucose through inhibition of glucose resorption in the proximal convoluted tubule, this class of medication has wide-ranging effects in the body, including acting as a mild diuretic, increasing sodium and water excretion and reducing blood pressure.13 Their use in patients with CKD and diabetes is associated with fewer cardiovascular events, reduced mortality and slowing of decline in GFR; consequently, SGLT2 inhibitors are now considered second-line agents in type 2 diabetes, after metformin.14
Reducing cardiovascular risk
Cardiovascular disease is the most frequent cause of death in people with early stages of CKD, for whom the absolute risk of cardiovascular events is similar to that of people who have established coronary artery disease. Reduced GFR and elevated urinary albumin are both independent risk factors for cardiovascular disease.11 Interventions to reduce this elevated risk include lifestyle change and blood pressure control, as discussed previously in this article.
Statins also play an important part in cardiovascular risk reduction. Dyslipidemia is common in patients with CKD and typically includes increased triglyceride levels in conjunction with decreased levels of high-density lipoprotein. Cholesterol may not be elevated, except in patients with marked proteinuria (nephrotic syndrome).15 These abnormalities likely contribute to the increased risk and may be partially reversed by a statin. A 2014 Cochrane review found that statins consistently lower the risk of death and major cardiovascular events by approximately 20% in people with CKD who are not receiving dialysis. The effects on stroke and kidney function were uncertain. The review concluded that statins have an important role in primary prevention of cardiovascular events and mortality in people who have CKD.16 National and international guidelines recommend statin or statin/ezetimibe combination for all patients living with CKD and aged ≥50 years, and for younger patients who have additional risk factors for cardiovascular disease.2,17
Other complications of chronic kidney disease
Although CKD can be caused by a variety of underlying pathologies, as it progresses, a consistent pathophysiological syndrome emerges. This is characterised by hypertension, cardiovascular disease, anaemia, mineral and bone disorder, volume overload and metabolic acidosis.18
Hypertension and increased cardiovascular risk are discussed previously in this article. Anaemia becomes increasingly common at lower levels of GFR. Interstitial cells in the renal cortex are the main source of erythropoietin in adults; patients with CKD have a relative deficiency in erythropoietin production.19 Erythropoiesis depends on adequate iron stores, and iron deficiency is common in CKD. An important cause is reduced iron absorption from the gut and reduced release of iron from storage in macrophages and the liver; these effects are mediated by hepcidin, a protein produced by the liver that is often elevated in patients with CKD. Other contributors to anaemia in CKD may include poor diet, blood loss and reduced erythrocyte survival.
Anaemia should always be investigated and not attributed to CKD without exclusion of other causes. Vitamin B12 and/or folate deficiency may contribute, as may hypothyroidism or hyperparathyroidism. Iron studies may be misleading as CKD is an inflammatory state and therefore serum ferritin may be elevated as an acute phase reactant. Iron deficiency can only be excluded if ferritin is >100 μg/L and the transferrin saturation is >20%.19 Patients with CKD and anaemia should be prescribed iron, orally or parenterally, to maintain these levels. If their haemoglobin remains below 100 g/L, they should also receive an erythropoietin analogue. Gastrointestinal bleeding should always be considered a possible cause of iron deficiency anaemia and the patient referred for endoscopy.
Mineral and bone disorder in CKD is characterised by altered calcium, phosphate, parathyroid hormone and vitamin D homeostasis. The consequences include diminished bone strength and mineralisation (renal osteodystrophy), soft tissue and vascular calcification, and accelerated progression of cardiovascular disease.20,21 The changes of mineral and bone disorder typically start to occur once GFR falls below 60 mL/min/1.73m2. Metabolic acidosis may develop later and further exacerbate calcium loss from bone.
Elevated serum phosphate, mainly due to reduced urinary excretion, occurs early in the evolution of mineral and bone disorder. The kidney is responsible for converting calcidiol (25-hydroxycalciferol) to calcitriol (1,25-dihydroxycalciferol), the most potent form of vitamin D. Lower levels of calcitriol impair vitamin D–dependent calcium absorption from the gastrointestinal tract. Elevated phosphate and lower calcium stimulate parathyroid hormone production (secondary hyperparathyroidism), and elevated parathyroid hormone increases bone turnover.20,21 Fibroblast growth factor 23, a protein secreted by osteocytes, appears to play a key part in these processes, in part by reducing renal conversion of calcitriol.22 Fasting levels of phosphate, calcium and parathyroid hormone should be monitored in all patients with CKD stage 3 or higher, and vitamin D deficiency corrected. Management of mineral and bone disorder is complex and should be undertaken in consultation with a nephrologist.2,23
Other complications emerge as CKD progresses, especially once GFR falls below 30 mL/min/1.73m2. Although such patients should normally be cared for in partnership with a nephrologist, general practitioners continue to have a key role in supporting the patient and their family; providing advice and information; encouraging healthy diet, exercise and other behaviours; coordinating management; offering preventive care; and screening for and addressing comorbidities.2
Key points
- CKD is not a diagnosis; attempts should always be made to ascertain the underlying pathology as this helps guide treatment.
- Strategies to delay progression of CKD include lifestyle advice, prevention of acute kidney injury, control of blood pressure and albuminuria, and disease-specific interventions.
- Inadvertent use of nephrotoxic medications is a common cause of acute kidney injury.
- Strategies to reduce cardiovascular risk in CKD include healthy lifestyle, blood pressure control and statins.
- Patients with renal anaemia, mineral and bone disorder, or advanced disease should normally be cared for in partnership with a nephrologist.