Infantile haemangiomas (IHs) are the most common benign vascular tumour of childhood, and they affect up to 10% of infants.1 They arise in the first few weeks-to-months of life2 and are clinically diagnosed. Risk factors include female sex, breech delivery, amniocentesis, Caucasian ethnicity, premature birth, low birthweight and advanced maternal age. IHs can be classified according to their subtype: superficial IHs are bright red plaques or papules, deep IHs are raised nodular lesions and mixed IHs are a combination of the two. IHs can also be classified according to their clinical distribution. Localised IHs are well-defined focal lesions, segmental IHs occur on one side of the midline and are usually more than 5 cm in diameter, multifocal IHs are multiple focal lesions, and indeterminate IHs are neither segmental or focal. Differentials for IHs include congenital haemangiomas, which are present at birth; pyogenic granulomas; tufted angiomas; and vascular malformations that do not show any signs of regression and grow in proportion with the child.
Most IHs are usually small superficial lesions that spontaneously resolve and parents require only reassurance and education. Some of these lesions are high risk, and up to 10% can cause complications including ulceration, airway obstruction, functional impairment or disfigurement.3 In this situation, treatment is initiated with oral or topical β-blockers, most commonly oral propranolol, and monitored closely. As a result of the various systems that high-risk IHs can affect, infants with high-risk IHs require multidisciplinary care. The general practitioner (GP) is in an ideal position to identify high-risk IHs during the six-week baby check, initiate investigations and be part of the multidisciplinary team to manage these lesions.
The aim of this article is to outline a strategy to identify high-risk IHs in the primary care setting, initial investigations to consider and when to refer using recently published guidelines from European,3 Australasian4 and American expert groups.5
IHs appear within the first few weeks of life and have three distinct phases. The proliferative phase involves rapid growth and occurs, on average, in the first nine months from onset.6 Superficial IHs reach their maximum growth within this time frame, but deep IHs can continue to grow for 18 months or more. The proliferative phase is followed by plateau, where the lesion is stable for months, and then the involution phase, where the lesion slowly decreases in size, leaving behind excess fibrofatty tissue. Traditional teaching suggests that resolution increases by 10% every year after the age of five years (ie 50% chance of resolution at five years, 70% at seven years); however, recent evidence suggests IHs may not significantly improve after 3–4 years of age,6 and therefore waiting for resolution after this age is not recommended.
IHs are considered high risk if they can cause life-threatening complications or functional impairment, have a risk of ulceration/disfigurement or are associated with clinically significant structural anomalies (Figure 1). Patients with high-risk lesions should be referred to a specialist clinician with experience in treating IHs depending on the specific risk involved (Table 1). For example, a lesion with the potential for airway compromise should be managed in a multidisciplinary setting with input from dermatologists; ear, nose and throat (ENT) surgeons; and paediatricians. According to guidelines, referral should occur as early as possible;6 an ideal time would be during the six-week baby check when this issue might be identified. The mean presentation to a dermatologist in one study was at five months of age, at which point the lesion may have significantly grown; earlier referral could prevent many of the aforementioned complications by allowing earlier treatment.2 GPs are in an ideal position to communicate with the relevant specialist staff and ensure patients can be seen urgently to treat high-risk lesions before they reach their maximum growth potential.
| Table 1. Investigation and management of high-risk haemangiomas |
| High-risk categories (% prevalence where known) |
Clinical findings |
Investigations to consider initiating* |
Disciplines involved |
Management options† |
| Life-threatening complications |
Lower face IHs (risk of airway obstruction) |
Lateral neck X-ray
|
ENT surgeon
Dermatologist |
ENT examination
Propranolol |
| Five or more cutaneous IHs (risk of liver haemangioma, heart failure, hypothyroidism) |
US liver
Thyroid function tests |
Dermatologist
Paediatrician
Cardiologist |
Propranolol
|
| Functional impairment |
Eye involvement |
|
Dermatologist
Ophthalmologist
Paediatrician |
Propranolol
|
| Lip and mouth involvement |
|
Dermatologist
Paediatrician |
Propranolol
|
| Ulceration (16%) |
High-risk areas – neck, intertriginous areas, lip, extremities
High-risk types – segmental |
|
Dermatologist
Paediatrician |
Propranolol
Pain management and dressings
Surgery
Laser therapy |
| Disfigurement |
High-risk areas – face, scalp, nasal tip, lip, ears, breast
High-risk types – segmental |
|
Dermatologist
Paediatrician |
Surgery
Propranolol
|
| PHACE syndrome |
Large segmental facial IH |
MRI
Magnetic resonance angiography
Electrocardiography
Transthoracic echocardiography |
Paediatrician
Dermatologist
Cardiologist
Neurologist |
|
| LUMBAR syndrome |
Large segmental lumbosacral IH
Sacral dimple, tuft of hair,
skin tag |
US spine
US abdomen
US pelvis and MRI spine |
Paediatrician
Dermatologist
Neurologist |
|
| PELVIS syndrome |
Large segmental perineal IH with associated genetic deformation |
US spine
US abdomen
US pelvis and MRI spine |
Urologist
Dermatologist
Neurosurgeon
Paediatric neurologist |
|
*Investigation for functional impairment, ulceration and disfigurement would be guided by the relevant specialties and is beyond the scope of this article.
†Management for PHACE, LUMBAR and PELVIS syndromes would be guided by the relevant specialties and is beyond the scope of this article.
ENT, ear, nose and throat; IH, infantile haemangioma; MRI, magnetic resonance imaging; US, ultrasonography |
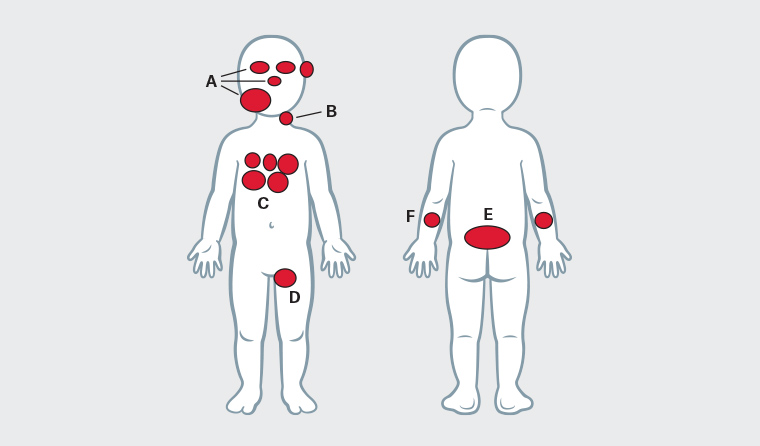
Figure 1. High-risk infantile haemangiomas (IHs)
A. IHs involving the lower face increase risk of airway obstruction and may indicate underlying PHACE syndrome; IHs involving the nose, lips and ears are at increased risk of ulceration and disfigurement; IHs involving the eyes increase risk of vision impairment and disfigurement; B. Neck haemangiomas have an increased risk of ulceration and may indicate underlying PHACE syndrome; C. Five or more haemangiomas indicate an increased risk of liver haemangiomas, cardiac failure and hypothyroidism; D. IHs involving intertriginous areas are at higher risk of ulceration; E. Lumbosacral IHs may indicate underlying spinal dysraphism; F. Segmental IHs on extremities are at high risk of ulceration.
Life-threatening complications
Segmental IHs of the lower face or anterior neck increase the risk of obstructive airway complications (Figure 2), especially when larger than 5 cm. Infants may present with early respiratory symptoms such as stridor and barking cough that could be easily mistaken for inflammatory croup. The mean time for diagnosis of obstructive complications is at four months of age.5 Infants with these IHs should be referred to a dermatologist and ENT surgeon as soon as possible. It is important to note that up to half of infants with airway compromise may not even have visible cutaneous IHs.7 Therefore, healthcare providers and parents must be vigilant and ensure immediate emergency medical attention is sought if an infant develops increasing signs of respiratory distress.
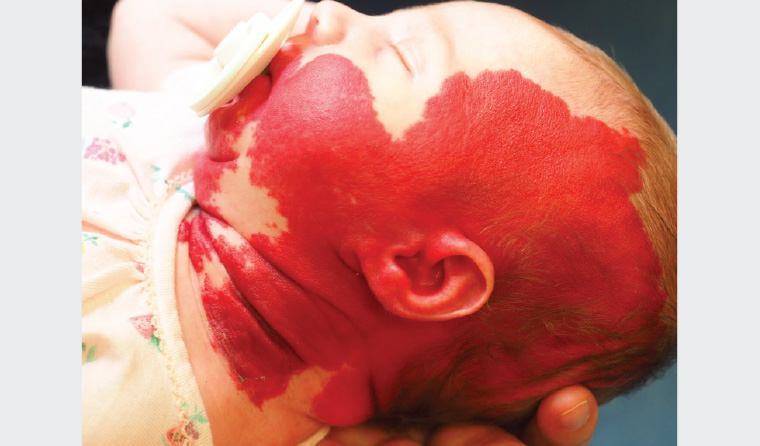
Figure 2. Large segmental facial haemangioma in a patient with PHACE syndrome
Image courtesy of Dr Deshan Sebaratnam
Having five or more cutaneous IHs increases the risk of having hepatic haemangiomas, high-output cardiac failure and hypothyroidism. Hepatic haemangiomas are usually focal, asymptomatic and require no treatment; however, some may cause high-output cardiac failure because of macrovascular shunting. Signs of cardiac failure include tachypnoea, increased work of breathing, difficulties feeding and failure to thrive. Higher-risk ‘diffuse’ hepatic haemangiomas can result in hepatomegaly severe enough to cause abdominal compartment syndrome and haemodynamic instability before the age of four months.8 Hypothyroidism can occur as a result of inactivation of thyroid hormones by type-3-iodothyronine deiodinase in IH tissue.9 Affected infants may have intermittent bradycardia, hypothermia, reduced muscle tone, failure to thrive, jaundice and constipation. As a result of these risks, screening liver ultrasonography and thyroid hormone testing is recommended for infants with five or more IHs.10 These infants should be referred to a dermatologist and paediatrician.
Functional impairment
IHs around the eyes can cause uniocular visual disturbance because of mechanical ptosis, strabismus, anisometropia and astigmatism.11 This can lead to amblyopia, and prompt referral of an infant with ocular involvement to a dermatologist and ophthalmologist can prevent these issues. IHs on the lips or around the mouth (Figure 3) can lead to feeding issues and failure to thrive and should be referred to a paediatrician and dermatologist for management.5
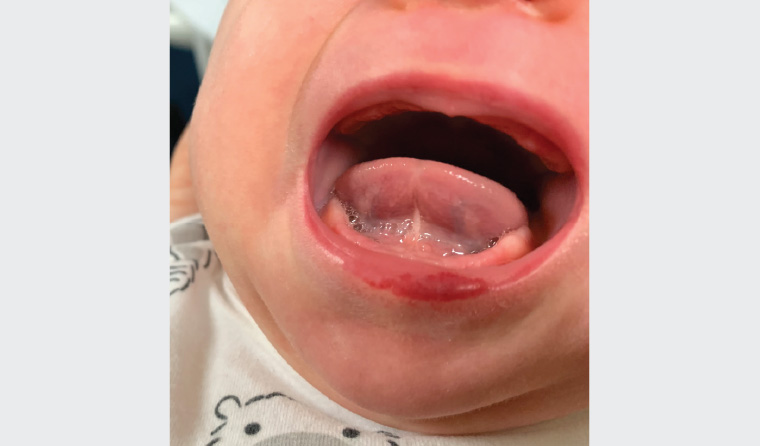
Figure 3. Infantile haemangioma affecting the lower lip
Image courtesy of Professor Gayle Fischer
Ulceration, disfigurement and scarring
Ulceration can occur in up to 15.8% of infants,12 and risk factors include the type of IH, its location and size. High-risk subtypes include segmental IHs, and high-risk locations include the neck, face, lips, extremities (Figure 4) and intertriginous areas (axillae, inguinal region, perineum, perianal) because of friction and maceration. Higher-risk sizes are those greater than 2 cm (in infants aged three months or older) or greater than 1 cm in size (in infants aged less than three months).6 Bleeding from ulceration is usually minor and manageable with pressure; however, there are rare cases of life-threatening blood loss reported.13 Disfigurement and scarring occur because of the potential size of the IHs, and high-risk areas include the nose, central face (if the IH is 2 cm or larger) and ears. Breast involvement in female infants can cause permanent changes in breast development. Although disfigurement and scarring may not be functionally impairing, they can cause significant psychological and social issues and should be managed appropriately.14
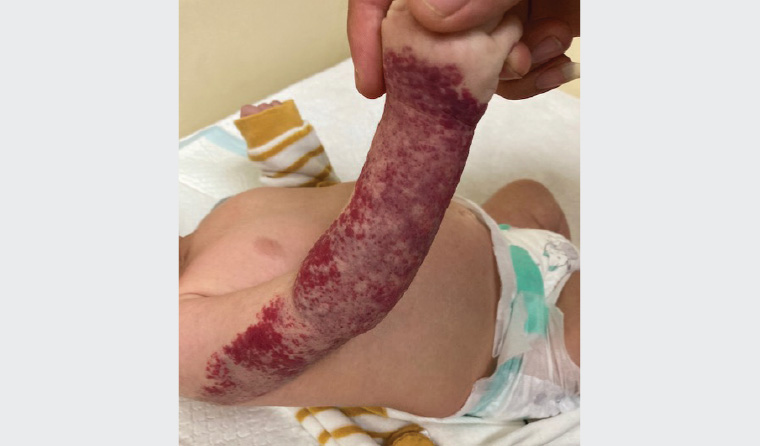
Figure 4. Large segmental haemangioma affecting the whole right extremity – high risk of ulceration
Image courtesy of Dr Deshan Sebaratnam
Structural syndromes
Patients with segmental IHs that involve the face, scalp or neck have an increased chance of having PHACE syndrome; one study has shown 30% of patients with segmental IHs involving the lower face have this syndrome.15 PHACE is an acronym for a syndrome that is characterised by Posterior fossa brain malformations, large segmental Haemangiomas affecting the face, Arterial anomalies including coarctation of the aorta, cardiac anomalies, Cerebrovascular abnormalities and Eye abnormalities.16 Patients with segmental IHs that involve the face should be investigated with magnetic resonance imaging (MRI) or magnetic resonance angiography of the head and neck, electrocardiography and transthoracic echocardiography to assess cardiac involvement.5
Segmental IHs involving the lumbosacral region are associated with LUMBAR syndrome, which includes Lower body haemangioma, Urogenital anomalies, Myelopathy, Bony deformities, Anorectal malformations and Renal anomalies.17 Segmental IHs involving the perineum are associated with PELVIS syndrome, which includes Perineal haemangioma, External genital malformations, Lipomyelomeningocele, Vesicorenal abnormalities, Imperforate anus and Skin tags.18 These syndromes should be investigated with ultrasonography of the abdomen, pelvis and spine, and MRI.19
Management
Most simple IHs require only education, support and ongoing monitoring. Worried parents should be encouraged to schedule frequent visits with the GP, take photographs and contact the GP if concerned regarding a lesion’s appearance, unexpected rapid growth, ulceration, bleeding or pain. The role of dermatologists and paediatricians is to assess referred high-risk IHs (Figure 5), commence appropriate treatment, follow up progress and correspond with the GP who will be caring for the infant in the community.
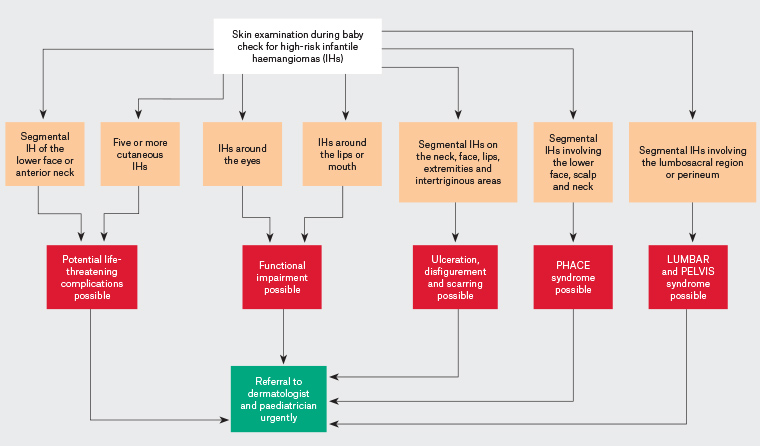
Figure 5. Flow diagram for the assessment and referral of high-risk infantile haemangiomas. Click here to enlarge
Oral propranolol is considered a first-line agent when IHs are high risk or develop complications.20 Its precise mechanism of action is unknown, and adverse effects include hypoglycaemia, sleep disturbances, bronchial irritation, bradycardia and hypotension.5 Oral propranolol requires vigilant monitoring to ensure significant adverse effects are not missed; a potential alternative is atenolol, which might be as effective and better tolerated but has less evidence for its application.21,22 A meta-analysis has shown that propranolol has a clearance rate of 95% (complete or nearly complete resolution).23 Where β-blockers are poorly tolerated, contraindicated or not effective, oral corticosteroids can be used and have a clearance rate of 43%.23 Adverse effects of oral steroids include cushingoid appearance, infections, growth retardation and hypertension.5 Topical timolol is a treatment option for superficial IHs and has a clearance rate of 64%.23 There is less evidence for this treatment option, and its adverse effect profile is promising but has not yet been clearly established. Wound care should also be used in ulcerated haemangiomas with wound cleaning, dressings and adequate analgesia.24 Surgical intervention can be considered for solitary IHs when they are refractory to pharmacotherapy and develop complications including ulceration or obstruction or have the potential for disfigurement. Pulsed dye lasers have been shown to be superior to other lasers and are used to treat some IHs.25 They have been advocated for treatment of ulcerated lesions and early proliferative lesions; however, the evidence comes mainly from case reports, and some controversy exists regarding increased risk of scarring and hypopigmentation.6
Conclusion
Although most IHs seen in clinical practice will be benign and self-limiting, it is important that clinicians are vigilant in identifying high-risk IHs and potential complications. IHs have significant interpatient variability; even within the same patient, multiple IHs may have significantly different characteristics and growth patterns. GPs are ideally placed to identify high-risk IHs, monitor therapy and monitor low-risk IHs. A skin check for IHs can be easily incorporated into the six-week baby check while assessing all the other facets of this examination such as the abdominal, genital and neurological examinations. Worried parents should be encouraged to actively monitor lesions, take photographs and liaise with their GPs with concerns regarding lesion growth, ulceration, bleeding or pain. Patients with high-risk IHs should be referred to dermatologists as early as possible, as this will ensure management can be commenced prior to the maximum growth of the lesion and prevent potential complications. Where this is not possible, such as in geographically isolated regions, telemedicine could be used to ensure appropriate guidance is sought.
Key points
- IHs are usually small, superficial, self-limiting lesions that may require no further investigation or intervention.
- High-risk IHs are those that have possible life-threatening complications; risk of ulceration, disfigurement or functional issues; and may be associated with underlying structural syndromes.
- GPs are ideally placed to identify high-risk IHs during routine baby checks.
- Early referral is key to optimising management of high-risk lesions.
- Infants with respiratory symptoms and IHs on the lower face should be immediately referred to the emergency department for acute airway management.
- High-risk types of haemangiomas are segmental, and high-risk locations include the face (nose, lips, ears, lower face), scalp, neck, intertriginous areas, extremities and lumbosacral area.