Melasma (also referred to as chloasma or the mask of pregnancy) is a common, acquired cutaneous pigmentary condition that results in mostly symmetrical, brownish pigmentation that affects the face and occasionally the forearms and back (Figure 1A).1,2 The global prevalence of melasma is approximately 1%, although higher prevalence rates have been reported in people with darker Fitzpatrick skin types (III–V), particularly in people of Latin American (9–30%), South-East Asian and South Asian (approximately 40%) ethnicity.3 Women are more likely to develop the condition than men, with the average age of onset between 20 and 40 years, although it can commence in adolescence and is thought to be mediated by hormonal factors.3,4 As a result of melasma frequently involving the face and the therapeutic challenge in management, the condition has a significant psychosocial impact on patients, with patients reporting shame, reduced self-confidence, anhedonia and a negative influence on interpersonal relationships and work productivity.4
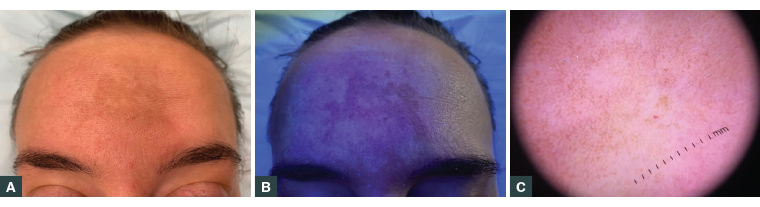 Figure 1. Epidermal melasma
A. Hyperpigmentation of the forehead; B. Wood’s lamp examination showing an accentuation of pigment; C. Dermoscopic examination revealing a pigmented brown reticular network with telangiectasia
Figure 1. Epidermal melasma
A. Hyperpigmentation of the forehead; B. Wood’s lamp examination showing an accentuation of pigment; C. Dermoscopic examination revealing a pigmented brown reticular network with telangiectasia
Treatment often requires a multimodal approach that targets pigment production to achieve equilibrium and disease remission.1 As a result of the high rate of recurrence, maintenance treatments are often essential, with strict light protection required to prevent relapses that characterise this chronic condition.
Pathophysiology of melasma
Although the pathogenesis of melasma remains unclear, it has been recognised as a disorder of photoageing. There have been several aetiological factors identified as leading to the release of vascular mediators that stimulate angiogenesis and the subsequent activation of melanocytes.2
Factors implicated include the following:
- Ultraviolet (UV) light – UV light can penetrate the epidermis of the skin and is thought to induce reactive oxygen species and promote melanin production (melanogenesis) in the skin.5 The shorter wavelengths of visible light (such as blue and purple light) have also been shown to induce long-lasting hyperpigmentation, particularly in darker skin types.6 Cumulative sun exposure can cause increased pigmentation in the skin that often persists long term.
- Family history – this is an important risk factor for developing melasma, with some studies reporting that up to approximately 60% of patients with this condition have a positive family history, suggesting a genetic predisposition.3,7
- Hormonal influences – oestrogen and progesterone may be implicated in the development of melasma, particularly given the increased prevalence of melasma during pregnancy, with the use of oral contraceptive agents containing oestrogen and progesterone, menopausal hormone therapy, intrauterine devices and implants.2,8,9 The activities of oestrogens and progesterones are mediated by specific oestrogen and progesterone receptors expressed in human skin. These have been implicated in a quarter of affected women.3 Extrafacial melasma on the forearms has been associated with the perimenopausal state and the use of topical oestrogen replacement therapy.7 Oestrogen is thought to be a more important mediator than progesterone.
- Medications – ingredients found within some perfumed soaps and cosmetic products may cause a phototoxic reaction that can activate the onset of melasma.10 Systemic agents including anti-epileptic, antimalarial, antipsychotic and cytotoxic/antineoplastic medications have been reported to potentially induce hyperpigmentation.11
- Heat exposure – it has been suggested that extended occupational heat exposure or exposure to cooking fires may be linked to the development of melasma through thermal and UV damage.12
Melasma may be associated with endocrinological conditions such as thyroid disease. Serum levels of thyroid stimulating hormone, antithyroid peroxidase and antithyroglobulin antibody were noted to be elevated in patients with melasma when compared with those without melasma.13
It has been suggested that multiple pathways – including the melanocyte-stimulating hormone/cyclic adenosine monophosophate, KIT and Wnt pathways – are implicated in the upregulation of tyrosinase and microphthalmia-associated transcription factor, resulting in the stimulation of melanogenesis and the development of melasma.8
Clinical manifestations
The characteristic appearance of melasma is that of bilateral light-to-dark brown asymptomatic macules distributed on the malar cheeks, forehead, upper lip and/or mandible with an irregular border. This typical appearance means that the diagnosis is usually straightforward and can be made clinically. Clinical examination using a Wood’s lamp that emits black light (UVA1) and dermoscopy may be used to aid in diagnosis and determining the level of melanin deposition (Figures 1B and 1C).10
There are several distinct patterns (Figure 2), including:14
- centrofacial – located on the cheeks, nose, forehead and upper lip (excluding the philtrum) and is present in approximately 50–80% of cases
- malar – located over the nose and malar cheeks
- mandibular pattern – located over the mandible and chin
- extrafacial – this pattern is variable, but is predominantly located on the upper extremities, often on sun-exposed sites.
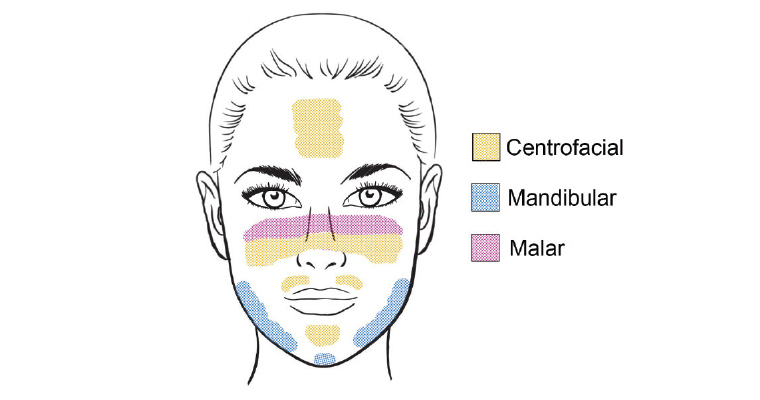
Figure 2. Clinical patterns of facial melasma
Reproduced with permission from Gupta M, Doolan BJ, Manungo F, Diagnosis of melasma and diagnostic tools, Opinions & Progress in Cosmetic Dermatology 2020;1:15–18.
An additional variation is erythematous or inflamed forms of melasma (erythrosis pigmentosa faciei).10
Melasma is sometimes divided into epidermal, dermal and mixed types on the basis of the level of melanin deposition in the epidermis (epidermal melanosis) and/or the dermis (dermal melanosis).10 The level of pigment can be identified with a combination of clinical, Wood’s lamp and dermoscopic findings by an experienced dermatologist.
A Wood’s lamp is a cheap, non-invasive and relatively easy to use device that can help determine the level of skin pigment. By emitting UV light (320–400 nm) in a dark room, hyperpigmented skin will be enhanced, revealing border contrast and variation in fluorescence.15 Furthermore, epidermal melasma usually displays accentuation under light, while dermal melasma shows none.16 Dermoscopic evaluation of epidermal melasma shows predominantly brown colour, with scattered islands of brown reticular network with fine dark granules scattered on the surface. Dermoscopic features of dermal melasma include a brown-to–ash grey colour with a more uniform involvement with no areas of pigmentation sparing and a reticuloglobular pattern, telangiectasia and archiform structures.16 Mixed melasma shows features of both epidermal and dermal subtypes.
Reflectance confocal microscopy can be used to evaluate melasma on a cellular level. A biopsy of the skin may be completed to confirm the diagnosis of melasma or, more often, to rule out other differential diagnoses.
Differential diagnosis
There are many skin conditions that have similar features to those of melasma and may clinically overlap. The most common differential diagnoses for melasma are listed in Table 1.17
| Table 1. Differential diagnosis of melasma17 |
| Condition |
Age of onset |
Location |
Clinical presentation |
| Acanthosis nigricans |
Any age |
Malar region; tends to spare the zygoma |
Symmetric hyperpigmented plaques; past/family history of diabetes and/or obesity |
| Acquired dermal macular hyperpigmentation |
Any age, but more common at 30–40 years |
Face, neck and proximal extremities |
Raised erythematous margins, occasional moderate pruritus with some hypopigmentation on a greyish background |
| Actinic lichen planus |
Any age |
Sun-exposed sites: face, neck and backs of the hands |
Erythematous to violaceous flat-topped papules
that are pruritic and polygonal; more common in darker skin |
| Discoid lupus erythematosus |
Any age |
Malar and periorbital regions |
Patches of facial skin atrophy with annular, mildly elevated erythema |
| Medication-induced pigmentation* |
Any age |
Usually face and arms |
Slate-grey colour ± involvement of other regions
(eg shins, scar sites, mucosal membranes) |
| Ephelides (freckles) |
Any age |
Sites of sun exposure |
1–10 mm well-demarcated hyperpigmented
round/oval macules |
| Frictional melanosis |
Any age |
Any site |
Due to excessive, repeated mechanical stimulation (ie habit of rubbing) |
| Hori’s macules |
20–70 years |
Forehead, temples, upper eyelids and alar of nose |
Bilateral and symmetrical small, greyish brown to blue-grey spots on the prominence of the cheeks and less often the temples, nose, eyelids, and forehead; mainly affects Asian women |
| Naevus of Ota |
Infancy to adolescence |
First two branches of the trigeminal nerve |
Blue-to-grey speckled or mottled coalescing macules or patches affecting the forehead, temple, malar area or periorbital skin; usually unilateral |
| Ochronosis (exogenous) from hydroquinone use |
Any age |
Sites of prior hydroquinone use |
Blue-black hyperpigmentation |
| Poikiloderma of Civatte |
30 years and older |
Sides of neck, lateral cheeks and upper chest |
Linear telangiectasia, erythema, mottled hyperpigmentation, and superficial atrophy in a reticular pattern, symmetrically affecting sun-exposed areas; more common in women than men |
| Post-inflammatory hyperpigmentation |
Any age |
Any site |
Prior history of erythema or rash in area |
| Solar lentigines |
Any age |
Sites of sun exposure |
1–20 mm well-demarcated hyperpigmented
round/oval macules |
| *Includes medications such as minocycline, amiodarone, antimalarials, heavy metals, antiretrovirals, antipsychotics, clofazimine and imatinib |
Treatment
Melasma can be slow to respond to treatment, particularly if the condition has been present for many years.10 General measures for melasma treatment are listed in Box 1. Poor prognostic factors for melasma treatment include:
- Fitzpatrick skin types III–V
- genetic and familial predisposition
- long-standing disease ≥2 years persisting despite treatment
- history of procedural interventions (eg lasers, microneedling)
- history of treatment by ≥2 physicians (possibly suggesting long duration and recalcitrant disease)
- long-term self-treatment with topical steroids
- ochronosis from hydroquinone use – either long term or high strength
- mixed-type melasma.
| Box 1. General measures for melasma management |
- Identify factors that trigger the patient’s signs and symptoms.
- Prioritise year-round lifelong light protection, including against visible light.
- Wear a broad-brimmed hat on sunny days when outdoors.
- Decrease occupational/domestic heat and light exposure.
- Use broad-spectrum very high protection factor (SPF 50+) sunscreen with protection from ultraviolet A (UVA), UVB and visible light, applied to the whole face daily, year-round. Sunscreens containing iron oxides are preferred, as they screen out some visible light as well as ultraviolet radiation. All tinted makeup products (creams/powders) contain iron oxide.
- Use a mild cleanser and, if the skin is dry, a non-perfumed moisturiser.
- Cosmetic camouflage (makeup) is invaluable to disguise the pigment. Concealers can be used as a thick, more opaque foundation makeup. Colour correctors can be used to tone down pigmentation.
- Consider discontinuation of hormonal contraception, if permissible.
|
The most common therapeutic agents used are those that inhibit the production of melanin by decreasing melanogenesis and melanocyte proliferation.18 Table 2 outlines detailed treatment options, including topical and systemic agents. Hydroquinone 2–5% as a cream or lotion, applied at night for 2–4 months, has been shown to result in significant improvement in melasma.18 Topical treatments including azelaic acid, kojic acid and tranexamic acid have been shown to significantly reduce melasma and should be considered prior to chemical peels or laser therapy.18 The most successful formulation has been Kligman’s formula, which is a combination of hydroquinone, tretinoin and dexamethasone in a cream base.1 This has been found to result in improvement or clearance in up to 60–80% of patients treated10 and is still considered the gold standard in the treatment of melasma.17 The additive use of a hat and broad-spectrum sunscreen in the treatment regimen is critical to the success of treatment of melasma and to minimise relapse. The treating specialist must also ascertain that there are no barriers to sunscreen use, such as irritation or problems with sunscreen acceptability, before commencing treatment. Oral tranexamic acid is also becoming more wildly used, given its low cost and ease of prescription in general practice. A recent meta-analysis of randomised controlled trials assessing the use of oral tranexamic acid for adult melasma showed significant efficacy and safety.19 Physicians should educate themselves and the patient regarding dosage, side effects and precautions before prescribing oral tranexamic acid. Topical use has not been found to be entirely effective.
| Table 2. Topical and systemic agents available for the treatment of melasma18 |
| Agent |
Mechanism of action |
Dosing |
Side effects |
Comments |
| Topical agents |
| Kligman’s formula (hydroquinone/ tretinoin/ dexamethasone) |
Inhibits tyrosinase; increases epidermal keratinocyte turnover; anti-inflammatory |
5%/0.025–0.05%/0.1% twice daily for three weeks
|
Skin irritation, erythema, telangiectasia, hyperpigmentation following irritant dermatitis |
The gold standard of treatment. Start infrequent application and titrate up as tolerated to minimise irritant dermatitis. Best to initiate treatment in the winter in Australia. Treatment duration is 3–4 weeks, followed by review for clinical efficacy. Maintenance therapy will be necessary with non-hydroquinone therapies or fixed triple combination intermittently, twice a week or less often. |
| Hydroquinone |
Inhibits tyrosinase |
2–5% daily |
Erythema, burning, itching, irritation and onchronosis with high doses or extended use |
Can be used in combination. Gold standard of treatment in combination therapy. Avoid in pregnancy. |
| Azelaic acid |
Inhibits tyrosinase |
5–20% twice daily |
Stinging, burning, pruritus and dryness |
Good response for treatment of epidermal melasma. |
| Kojic acid |
Inhibits tyrosinase |
1–2% daily |
Skin irritation, contact dermatitis and increased skin sensitisation |
Mixed results. |
| Ascorbic acid (vitamin C) |
Inhibits reactive oxygen species |
5–15% daily |
No significant adverse effects |
Well tolerated, but highly unstable; best in combination. |
| Cysteamine |
Inhibits tyrosinase |
5% daily |
No significant adverse effects |
Effective for treatment of epidermal melasma. |
| Metimazole (antithyroid medication) |
Potent peroxidase inhibitor; blocks melanin synthesis |
5% daily |
No significant adverse effects |
Avoid in pregnancy (especially first trimester). No adverse events in neonates during lactation. |
| Tranexamic acid |
Blocks binding of plasminogen to keratinocytes |
2–5% twice daily |
Erythema, scaling, dryness and irritation |
Improvement in melasma. |
| Glutathione |
Decreases tyrosinase to skew conversion of eumelanin to pheomelanin |
2% daily |
No significant adverse effects |
Inferior to hydroquinone and combination therapy. |
| Iron oxide sunscreens |
Blocks visible light |
SPF 50+ |
No significant adverse effects |
Well tolerated, showing improvement in mild melasma. |
| Tretinoin |
Increases epidermal keratinocyte turnover |
0.025–0.05% daily |
Irritant dermatitis with occasional contact dermatitis and photosensitisation |
Often used in combination. Inferior to combination therapy if used alone. Start with lower strength. |
| Corticosteroid treatments (hydrocortisone) |
Anti-inflammatory; nonselectively inhibits melanogenesis |
1% (variable) daily |
Telangiectasias, epidermal atrophy, steroid-induced acne, striae and hypopigmentation |
Work well to fade colour quickly and treat contact dermatitis, but potent steroids should be avoided on the face. |
| Systemic agents |
| Tranexamic acid |
Blocks binding of plasminogen to keratinocytes and decreases prostaglandin and vascular endothelial growth factors |
500–750 mg daily |
Headaches, hypomenorrhea, mild abdominal discomfort, and transient skin irritation
|
Approximately 10–40% reduction in melasma but high rate of relapse within two months of cessation. Tranexamic acid may increase thromboembolic risk, so it is important to assess patients. |
| Polypodium leucotomos extract |
Increases matrix metalloproteinase expression and increases cell structural integrity during ultraviolet damage |
240 mg three times daily |
No significant adverse effects |
Weak evidence for significant improvement in melasma. |
| Glutathione |
Decreases tyrosinase to skew conversion of eumelanin to pheomelanin |
500 mg daily |
Potentially severe adverse effects include possible liver, renal and thyroid dysfunction |
Variable results; only effective in some parts of the body and do not elicit long-lasting effects. |
| Carotenoids (lutein/zeaxanthin) |
Antioxidants; may inhibit lipid peroxidation and quench singlet oxygen |
10 mg/2 mg daily |
Skin discolouration, but reversible with discontinuation |
Significant improvement in melasma and reduces erythema. |
Procedural techniques used to treat melasma
When considering treatment for melasma, the rationale is to treat the affected skin while avoiding damage to normal, unaffected skin. Unfortunately, protection of normal pigmented skin while selectively targeting melasma is hard to achieve, and treatment often results in post-inflammatory hyperpigmentation. The use of chemical peels and lasers should be approached with caution, as they may exacerbate or cause a relapse of melasma.10 These interventions should be undertaken by an expert with an understanding of skin of colour.
Chemical peels with active ingredients such as alpha hydroxy acids (AHAs; eg 6–12% glycolic acid creams/lotions) and beta hydroxy acids (BHAs; eg salicylic acid) have also been shown to be useful in the treatment of melasma as they can remove surface skin and decrease the physiological activity of tyrosinase.20 A series of 3–6 peels may be required to show benefit. AHA and BHA peels can also be used for ‘spot peeling’ of discrete areas of hyperpigmentation and to reduce contrast between normal skin and melanotic macules.20
Intense pulsed light (IPL) systems emit a range of different wavelengths that provide penetration for epidermal and dermal melasma simultaneously.21 Treatment is best used in combination with topical agents. IPL therapy results in a modest improvement but has a moderate recurrence rate if not used with aggressive topical therapy. Q-switched lasers (neodymium-doped yttrium aluminium garnet), ablative and non-ablative fractionated resurfacing lasers and picosecond lasers can also be used for selective columns of microthermal damage and have been shown to reduce scars and epidermal injury but may also cause hyperpigmentation.10,21 Overall, laser treatment, when compared with medical modalities of treatment, carries a higher risk for relapse and risk for the condition becoming more resistant to treatment.10 Some techniques are associated with an increased risk of post-inflammatory hyper- or hypopigmentation and are best undertaken by experts in the field.
Cosmetic camouflage agents may be an important adjunct therapy for patients.17 Therapy should be chosen on the basis of availability, cost, ease of application, aesthetic acceptability and colour match.17 Patients may benefit from advice from an expert in camouflage cosmetics.
Conclusion
Pigmentation is a downstream result of the process of photoageing, vascular changes and resulting inflammatory mediators.12 Addressing the hyperpigmentation alone will not yield lasting benefits, hence combination therapy addressing photoageing, light protection and melanin pigment is required.
Results take time, requiring multiple sessions and ongoing maintenance. The condition is more amenable to treatment if treated early in the course of its evolution. Even after receiving adequate treatment, melasma may relapse on exposure to the summer sun (particularly in Australia) or because of changes in hormonal or endogenous factors.10 Thus, it is best that patients are counselled regarding the chronicity of the condition so that realistic treatment goals are achieved.
Key points
- Melasma is a common disorder of pigmentation with which patients present to general practice.
- The pathogenesis of melasma remains unclear, but it is recognised as a disorder of photoageing resulting in stimulation of melanin pigment.
- A combination of hydroquinone, tretinoin and a topical steroid is considered the gold standard in treatment.
- Systemic agents, chemical peels and laser therapy are alternative options for treatment.
- Melasma is a chronic condition requiring ongoing maintenance to minimise relapse.