This article is the first in a series of articles on important topics in neurology.
Migraine is a common neurological disorder, affecting over one billion people worldwide. The World Health Organization Global Burden of Disease study ranked migraine as the second leading cause of disability.1 Every year, 2.5% of patients with episodic migraine progress to chronic migraine, with associated impacts on quality of life and healthcare use.2 The key modifiable risk factors are effective preventive medication, avoidance of acute medication overuse, obesity, stress, depression and anxiety.1,2
Diagnosing migraine
Migraine is defined by the International classification of headache disorders, third edition (ICHD-3), as occurring with or without aura (Table 1). It is further characterised as either episodic or chronic, with chronic migraine comprising ≥15 headaches/month for three months, of which at least eight days have features of migraine.3
| Table 1. The International Classification of Headache Disorders, third edition (ICHD-3), diagnostic criteria for migraine with and without aura3 |
| Migraine with aura |
| A |
At least two attacks fulfilling criteria B and C |
| B |
One or more of the following fully reversible aura symptoms:
1. visual
2. sensory
3. speech/language
4. motor
5. brainstem
6. retinal |
| C |
At least three of the following six characteristics:
1. at least one aura symptom spreads gradually over ≥5 minutes
2. two or more aura symptoms occur in succession
3. each individual aura symptom lasts 5–60 minutes
4. at least one aura symptom is unilateral
5. at least one aura symptom is positive
6. the aura is accompanied or followed within 60 minutes by headache<.li> |
| D |
Not better accounted for by another ICHD-3 diagnosis |
| Migraine without aura |
| A |
At least five attacks fulfilling criteria B–D |
| B |
Headache attacks lasting 4–72 hours (untreated or unsuccessfully treated) |
| C |
Headache has at least two of the following four characteristics:
1. unilateral location
2. pulsating quality
3. moderate or severe pain intensity
4. aggravation by or causing avoidance of routine physical activity (eg walking or climbing stairs) |
| D |
During the headache, at least one of the following:
1. nausea and/or vomiting
2. photophobia and phonophobia |
| E |
Not better accounted for by another ICHD-3 diagnosis |
| Reproduced with permission of the International Headache Society from Headache Classification Committee of the International Headache Society (IHS), The International Classification of Headache Disorders, 3rd edition, Cephalalgia 2018;38;1–211. |
Tips and traps in diagnosis
For busy clinicians, the three-question ID Migraine questionnaire can be used to screen patients and has a sensitivity of 0.84 (95% confidence interval [CI]: 0.75, 0.90) and specificity of 0.76 (95% CI: 0.69, 0.83).4 The questionnaire asks whether patients have experienced the following with their headaches during the past three months:
- felt nauseated or sick in the stomach
- were bothered by light (or a lot more than when they do not have headaches)
- had limited ability to work, study or do what they needed to do for at least one day.
While migraine is a common condition, there are several mimics that should be considered in the diagnosis. Several secondary headaches can exhibit some migrainous features, or even partially respond to triptans, and it is important to use the SNNOOP red-flag list to consider them and choose appropriate investigations (Table 2). Headaches that are most commonly confused with migraine include:
- new daily persistent headache – differentiated by its unremitting nature and clearly remembered day of onset
- tension-type headache – differentiated by its lack of associated migrainous symptoms and severity of symptoms
- hemicrania continua – a strictly side-locked headache with autonomic features and marked response to indomethacin
- ‘sinus headache’ – although commonly diagnosed, it is rarely the cause of chronic headaches. In a cohort of 140 patients with migraine, >80% were initially misdiagnosed as having a sinus headache, leading to a 7.75-year delay in treatment.5
| Table 2. SNNOOP10 list of red and orange flags20 |
| Red flag |
Related secondary headache |
| Systemic symptom/fever |
Intracranial infection, carcinoid or phaeochromocytoma |
| History of neoplasm |
Metastatic disease |
| Focal neurological deficit |
Stroke, brain abscess or infection |
| Abrupt onset headache |
Subarachnoid haemorrhage, pituitary apoplexy, reversible cerebral vasoconstriction syndrome, haemorrhage, cranial or cervical vascular pathology |
| Onset after the age of 50 years |
Giant cell arteritis, neoplasm, mass lesion, vascular disorder, stroke |
| Change in pattern or recent onset |
Neoplasm, headaches from vascular or non-vascular disorders |
| Positional headache |
Intracranial hypertension or hypotension |
| Precipitated by sneeze/cough/exercise |
Posterior fossa malformation, Chiari malformation |
| Papilloedema |
Intracranial hypertension, mass lesions, venous sinus thrombosis |
| Progressive or atypical presentation |
Neoplasm, non-vascular disorder |
| Pregnancy or puerperium |
Postdural headache, pre-eclampsia, venous sinus thrombosis, hypothyroidism, diabetes, pituitary apoplexy, cranial or cervical vascular disorder |
| Painful eye/autonomic features |
Pathology in posterior fossa, pituitary or cavernous sinus, Tolosa–Hunt syndrome or ophthalmic cause |
| Post-traumatic |
Subdural haematoma or other vascular disorder |
| Pathology of immune system |
Opportunistic infection or metastasis |
| Painkiller overuse or new medication |
Medication-overuse headache or medication incompatibility |
Finally, migraine aura requires differentiation from a transient ischemic attack (TIA) and focal seizure. The presence of a headache is helpful but not diagnostic, as both can provoke headaches, and migraine auras can occur without a headache. Time course and progression is key; a TIA is of abrupt onset, does not progress and respects vascular territories. Although a focal seizure does ‘spread’ similarly to aura, the duration is seconds to minutes in contrast to an aura of 5–30 minutes.
Pathophysiology of migraine
There are four phases to a migraine attack: prodrome, aura, headache and postdrome, summarised in Figure 1. The prodrome, caused by hypothalamic activation, is associated with dopaminergic symptoms of yawning, fatigue and polyuria as well as altered food cravings. It can precede the rest of the migraine by hours to days.6
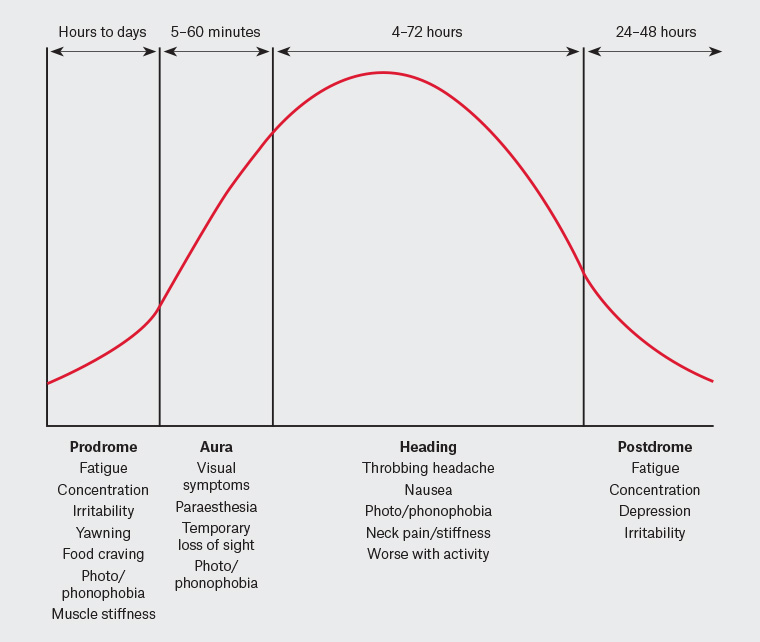
Figure 1. Phases of a migraine attack
One-third of patients experience an aura, caused by a spreading wave of cortical depolarisation, which gives the classical phenotypic ‘spreading’ description. Visual auras (photopsias, flickering lines) are most common, experienced by 65–99% of patients who have migraine with aura.6,7 Sensory, motor and speech aura symptoms may also be seen.
The trigger for the headache phase is still debated but involves activation of trigeminal afferents that innervate the dura as well as the trigeminal ganglion. Activation of these structures results in a release of vasoactive peptides including calcitonin gene-related peptide (CGRP), substance P, neurokinin A and pituitary adenylate cyclase-activating peptide (PACAP), causing local vasodilation. The trigeminal ganglion is a key structure as it exists outside the blood–brain barrier and is rich in CGRP and serotonin receptors, making it a therapeutic target. From here, nociceptive signalling is relayed to the trigeminocervical complex and through ascending connections to many other areas of the brain, contributing to the cognitive and autonomic symptoms of migraine.6
Finally, 60–94% of patients have symptoms following the headache, termed the postdromal phase. These include fatigue, mood disturbance and difficulty concentrating, which are likely continued from the prodromal phase.7 Patients may attribute these symptoms to side effects of their acute therapies; however, a meta-analysis has confirmed these as features of the disease.8
Treatment
Acute treatment
The goal of acute treatment is sustained pain freedom within two hours. It is recommended that treatment is commenced as early as practical and uses a combination approach (eg nonsteroidal anti-inflammatory drugs [NSAIDs], anti-emetic, triptan) if one therapy is ineffective. Choice of therapy will be tailored to comorbidity (eg triptans are contraindicated with cardiovascular disease); however, these authors recommend NSAIDs as first-line treatment (aspirin 900 mg or ibuprofen 600 mg), with the addition of triptans in patients who are non-responders. An illustrative case study is provided in Table 3.
| Table 3. Case study of a patient with episodic migraine |
| Baseline |
| Patient |
A woman aged 35 years with episodic migraine occurring 10 times per month. She experiences nausea and severe pain in the first 15 minutes of symptoms and has failed to respond to over-the-counter sumatriptan previously.
She has started a new office job and is skipping meals because of her current workload. She is having difficulty falling asleep because of some ruminating thoughts. |
| Clinician |
After making a positive diagnosis of migraine, addresses the various potentiating factors:
- Lifestyle management – counselling relaxation techniques, providing education on the interaction between fasting states and migraine, and the importance of regular breaks at work to maintain posture
- Acute management – commencing metoclopramide for nausea/analgesia, transitioning to a non-oral triptan as a result of the rapid onset of symptoms, and starting 900 mg aspirin
- Preventive management – commencing amitriptyline for dual benefit of migraine prevention and to aid in sleep, counselling expected response
|
| Three months later |
| Patient |
Returns having had a successful response to amitriptyline, lowering the number of migraines to on average four per month, after gradually up‑titrating the dose to 50 mg. She reports some mild morning sedation but otherwise tolerates the medication well and is sleeping better.
Her acute attacks are aborted after 30 minutes; however, a quarter of the time she notices that the pain comes back after 4–5 hours. She has had one prolonged migraine that was not responsive to her other agents and lasted three days. |
| Clinician |
Having seen a positive response to amitriptyline, encourages the patient to continue to take the medication, and tries to optimise the treatment:
- Lifestyle management – revisiting and reinforcing sleep hygiene
- Acute management – swapping the aspirin for naproxen sustained release, to try to avoid the ‘wearing-off’ effect of the triptan
- Preventive management – advising the patient to take the medication earlier in the evening, with dinner; if the sedation persists, reducing the dose to 35 mg and re-evaluating its efficacy
- Rescue therapy – discussing the option of a rescue therapy for a migraine that is not responsive to standard treatment, and giving the patient a script for prochlorperazine 25 mg per rectum
|
Tips and traps in acute treatment
In monotherapy, as a class, triptans will reduce headache pain within two hours for 42–76% of patients and relieve it in 18–50%.9 If a patient has only a partial response to triptans, rotating to a second agent is often successful (although combination therapy is superior to either therapy in isolation10). On meta-analysis, eletriptan and rizatriptan have the highest odds ratio for providing pain relief and therefore are worth trialling,11 while non-oral formulations such as wafers (rizatriptan) are useful for early nausea, and intranasal or subcutaneous formulations (sumatriptan) for nausea or rapid-onset symptoms.
For patients with a partial treatment response, addition of dopamine-blocking antiemetics can be effective. Dopamine pathways have a role in migraine pathophysiology, and consequently metoclopramide is effective in aborting a migraine attack, with intravenous metoclopramide being found to be superior to a nerve block in the emergency department.12 These authors recommend against the prescription of opioids, given the risk of medication overuse and tolerance.
All patients must be counselled about the risk of medication overuse headache, as overuse diminishes endogenous pain inhibition, increases neurogenic inflammation and leads to central sensitisation.13 The net effect is more frequent headaches, less responsive to both preventive and acute therapies, with an associated poorer quality of life.14 The ICHD-3 criterion for medication overuse is the regular overuse of analgesia for three months, the thresholds for which are:
- NSAIDs/paracetamol: >15 days/month
- triptans/opiates: >10 days/month (as discussed, opiate use for headache is not recommended).
For patients with status migrainosus that has not responded to standard acute therapies, there are several outpatient strategies. A two-week bridging course of naproxen sustained-release 750 mg or a short course of prednisolone (50 mg, weaning after three days) may be useful.15 Other strategies, including the use of neuroleptic and anti-epileptic therapies, depend on clinician confidence and familiarity;15 however, these authors use 25 mg of compounded prochlorperazine per rectum as a non-oral option in select patients.
Preventive treatment
Counselling and management of lifestyle risk factors for migraine are key components of effective migraine prevention, focusing on sleep, stress, exercise, diet, mental health and obesity. Identifying and minimising potential triggers to migraine are also important. Of interest, owing to hypothalamic activation during the prodromal phase, some symptoms (eg particular food craving) that patients experience prior to the onset of headache may be a symptom of the disease, rather than a trigger. Pavlovic et al recently performed a systematic review summarising triggers (including menstruation, sleep deprivation, fasting) and prodromal symptoms in migraine.16 Pharmacological treatment is indicated if individual attacks are refractory to acute treatment or there are more than four migraines/month. A migraine diary, examples of which are available on the Australian and New Zealand Headache Society website, is useful to monitor the burden of disease, medication overuse, response to preventive treatment and potential triggers. Patients should be counselled that a clinical response is judged after two months of treatment, with at least a 50% reduction of migraine days considered significant.
Tips and traps in preventive treatment
The relative efficacy of the common oral preventers was the subject of a meta-analysis in 2015, which found no significant difference.17 The choice of the first agent should therefore be based on tolerability and any possible secondary gain (eg weight loss with topiramate). Commonly used preventive medications are summarised in Table 4 to highlight the recommended therapeutic range and their regulatory status. Patients intolerant of higher doses may benefit from concomitant low-dose medication.
| Table 4. Preventive medications for migraine21,22 |
| Medication |
Dosage |
50% responder rate* |
Regulatory status |
Authors’ notes |
| Level A evidence – oral medications (EFNS or AAN) |
| Propranolol |
40 mg (increase at intervals of one week or greater [ie ≥1 weekly] by 40 mg to maximum 40–160 mg total daily dose [BD or TDS]) |
30–40%23,24 |
PBS: GB-M,
TGA: Yes |
Useful in anxiety, perimenopause; caution regarding mood and vivid dreams |
| Topiramate |
25 mg (increase ≥1 weekly by 25 mg to maximum 50–100 mg BD) |
46.3%25 |
PBS: Auth-M,
TGA: Yes |
Useful for weight loss |
| Sodium valproate |
200 mg (increase ≥1 weekly by 200 mg to maximum 200–600 mg BD) |
42%26 |
PBS: GB-O, TGA: No |
Avoid in women of childbearing age |
| Flunarizine |
5 mg (5–10 mg daily) |
58.6%27 |
PBS: No, TGA: SAS |
Use with caution in individuals with depression |
| Level A evidence – injectable medications |
| OnabotulinumtoxinA |
155 units, three times per month |
47.1% in CM17 |
PBS: Auth-M,
TGA: Yes |
|
| Erenumab |
140 mg, once per month |
41% in CM8 |
PBS: No, TGA: Yes |
|
| Fremanezumab |
225 mg, once per month |
47.7% in CM8 |
PBS: Yes, TGA: Yes |
|
| Galcanezumab |
240 mg, once per month |
27.6% in CM8 |
PBS: Yes, TGA: Yes |
|
| Level B evidence (EFNS or AAN) |
| Amitriptyline |
10 mg (increase ≥1 weekly by 10 mg to 25–75 mg daily) |
58.6%28 |
PBS: GB-O, TGA: No |
Useful for sleep/mood |
| Venlafaxine |
37.5 mg (increase ≥1 weekly by 37.5 mg to 75–150 mg daily) |
28%29 |
PBS: RB-O, TGA: No |
|
| Level C evidence (EFNS or AAN) |
| Candesartan |
4 mg (increase ≥1 weekly by 4 mg to
8–32 mg daily) |
40.4%30 |
PBS: GB-O, TGA: No |
Well tolerated |
| Gabapentin |
300 mg (increase ≥3 days by 300 mg,
900–3600 mg total daily [BD or TDS]) |
46.4%31 |
PBS: GB-O, TGA: No |
Can be useful during perimenopausal |
| Magnesium |
400 mg (400–600 mg daily elemental dose) |
– |
PBS: No, TGA: No |
Well tolerated |
| Coenzyme Q10 |
150 mg (150–300 mg daily) |
– |
PBS: No, TGA: No |
Well tolerated |
| Riboflavin |
400 mg (400 mg daily) |
– |
PBS: No, TGA: No |
Well tolerated |
| Other medications |
| Cyproheptadine |
4 mg (4–12 mg daily) |
– |
PBS: No, TGA: Yes |
Well tolerated |
| Melatonin |
2 mg (4–8 mg daily) |
54.4%28 |
PBS: No, TGA: No |
|
| Lamotrigine |
Pending interactions |
46%32 |
PBS: No, TGA: No |
Useful with prominent aura symptoms/mood |
| Nortriptyline |
10 mg (increase ≥1 weekly by 10 mg to 25–75 mg nocte) |
28.6%33 |
PBS: RB-O, TGA: No |
Useful where amitriptyline is not tolerated |
*The proportion of patients in a trial who had a 50% or greater reduction of migraine days
–, no data available; AAN, American Academy of Neurology; Auth-M, PBS authority for migraine; Auth-O, PBS authority for other indication; BD, bis in die (twice per day); CM, chronic migraine; EFNS, European Federation of Neurological Societies; GB-M, general benefit for migraine; GB-O, general benefit for other indication; nocte, at night; PBS, Pharmaceutical Benefits Scheme; RB-O, restricted benefit for other indication; SAS, Special Access Scheme; TDS; ter die sumendum (three times per day); TGA, Therapeutic Goods Administration |
For patients who have failed to respond to three oral agents, are not overusing acute analgesia and have chronic migraine, a neurologist may commence onabotulinumtoxinA (Botox) injections. The proportion of patients who have a 50% reduction in their migraine days with Botox following the Phase III REsearch Evaluating Migraine Prophylaxis Therapy (PREEMPT) protocol (the 50% responder rate) was 47.1%.18
Unfortunately, there is no panacea, and patients who have failed to respond to Botox, oral preventive medications and lifestyle measures are often managed collaboratively by their neurologists and general practitioners, and they may benefit from engagement with a chronic pain service. Revisiting the diagnosis, assessing any recent changes in lifestyle factors and considering combinations of previously tolerated therapies with partial response is often useful. Consideration may also be given to emerging therapies such as the use of a ketogenic diet, which, in a recent uncontrolled trial, remarkably reported patients with medication-resistant migraine transform from having chronic daily pain to pain only seven days/month, and warrants further study.19
Special scenarios
Medication-overuse headache, a common comorbidity to migraine, can be challenging to manage. Patient education regarding the condition and expectation management is key. Patients can expect a three-week worsening of their headaches when ceasing analgesia overuse. Bridging therapies, such as a two-week course of naproxen (for triptan or opiate overuse), may help reduce the severity while starting preventive treatment.
Where patients have menstrual-related migraine, ‘mini-preventive’ strategies may be employed if the patient has a regular cycle, such as regular naproxen or naratriptan starting 1–2 days prior to menses for five days. Choice of contraception requires an individualised discussion with patients, taking into account their individual risk of thrombosis, and continuous cycling of a contraceptive may be indicated in selected patients.
Emerging therapies
Several new therapies will emerge in Australia over the new few years for both acute and preventive treatment of migraine. The first of these are the CGRP monoclonal antibodies, which have reported positive phase II/III trials, of which subcutaneous fremanezumab and galcanezumab are listed on the Pharmaceutical Benefits Scheme (PBS), while erenumab has Therapeutic Goods Administration (TGA) approval. Patients are eligible to access these medications on the PBS if they have chronic migraine (≥15 headache days per month), have failed three preventative agents, are not receiving botulinum toxin on the PBS and are not overusing acute analgesia. These medications can only be started by a neurologist; however, the treating general practitioner may continue to prescribe them if the patient achieves a ≥50% reduction in headache days in the first three months.
As a medication class, CGRP monoclonal antibodies are well tolerated, with side effects of local injection-site reactions, constipation, fatigue and nausea. CGRP is widely expressed throughout the body, and any long-term off-target effect is not known. CGRP monoclonal antibodies may worsen hypertension, and caution is advised for use in patients with coronary or cerebrovascular disease. As a result of inadequate safety data, they should be avoided for patients who are pregnant or breastfeeding. CGRP monoclonal antibodies have a 50% responder rate of 45–60% in episodic migraine and 30–40% in chronic migraine. An intravenous monoclonal antibody, eptinezumab, has also been developed, but it is not yet registered with the TGA.8
Small molecule CGRP antagonists (gepants) are the second class of CGRP medication and are being developed as both an acute and preventive treatment for migraine. Several of these medications are now licensed with the Food and Drug Administration but not the TGA. They are well tolerated, with low rates of nausea and no evidence of liver derangement. Rimegepant, at a dose of 75 mg, achieves two-hour pain freedom in 20.6% of patients, and ubrogepant, at a dose of 50 mg, achieves pain freedom in 21.8%. A third agent, atogepant, is currently undergoing a phase III study in episodic and chronic migraine (NCT03777059/ NCT03855137).8
When to refer
Patients who have failed to respond to a therapeutic trial of 2–3 preventive medications, have a significant burden of symptoms or have comorbid medication-overuse headaches – or patients for whom the diagnosis is uncertain – should be referred for shared management with a neurologist with expertise in headache or a tertiary headache clinic.
Conclusion
Migraine is a common, disabling neurological condition; however, effective acute and preventive treatment significantly limits its disability. The combination of NSAIDs and triptans at the onset of an attack is more likely to be efficacious then either therapy individually. Preventive medication is indicated for patients with more than four attacks per month or if individual attacks are hard to treat, both to limit the morbidity of the disease and reduce the risk of medication overuse. Choice of preventive medication should be tailored to a patient’s comorbidities and potential side effects, and must be trialled for 6–8 weeks to assess efficacy.
Key points
- Abortive treatments for migraine are more effective if taken early and in combination.
- Overuse of abortive treatments causes medication-overuse headaches.
- Preventive treatments should be considered if patients have >4 migraines/month.
- The aim of preventive therapy is at least a 50% reduction in headaches after eight weeks.
- Preventive therapy should be based on preference, comorbidity and potential side effects.