Placental examination has a critical role in understanding adverse fetal and maternal outcomes in pregnancy. However, progress in correlating placental pathology with clinical phenotypes has been hampered by methodological issues in many studies published.1 Additionally, there appears to be a knowledge disparity between perinatal pathologists reporting microscopic changes seen in the organ and understanding of the clinical significance of placental histopathological diagnoses by obstetric care providers.2 When reported and interpreted correctly, the placenta provides useful information that may explain adverse pregnancy outcomes as well as guide further management of the mother, newborn or future pregnancy.3 Table 1 lists the most important and recurrent histopathological diagnoses of the placenta with some of the clinical steps that may be taken, based on expert opinion as well as anecdotal and limited scientific evidence available to date.4
| Table 1. Clinical significance of placental histopathological diagnoses |
| Placental diagnosis |
Suggested clinical action postpartum and in future pregnancy |
| Ascending intrauterine infection (acute subchorionitis, acute chorioamnionitis, acute fetal vasculitis, acute funisitis) |
- Correlate with microbiology, culture and sensitivity studies
- Consider or review antimicrobial treatment for mother and baby
- Cervical cerclage in future pregnancy if complicated by recurrent preterm labour
|
| Chronic inflammatory condition (chronic villitis, chronic deciduitis, chronic chorioamnionitis, chronic histiocytic intervillositis, eosinophilic T-cell vasculitis |
- Screen for viral, treponemal and protozoal (syphilis, toxoplasmosis, rubella, cytomegalovirus, herpes simplex [‘STORCH’]) infection
- Malarial screen if chronic histiocytic intervillositis predominant
|
| High-grade villitis of unknown aetiology (VUE) |
- Maternal autoimmunity screen
- Consider low-dose aspirin, prednisolone, intravenous immunoglobulin in future pregnancy
- Specialist obstetric care for possible fetal growth restriction with early delivery in future pregnancy if indicated
|
| Idiopathic chronic histiocytic intervillositis |
- As for VUE
- Also consider low molecular weight heparins, hydroxychloroquine in future pregnancy
|
| Massive perivillous fibrin deposition and maternal floor infarction |
- Consider pravastatin, low-dose aspirin, low molecular weight heparins, immunoglobulin
|
| Fetal vascular malperfusion |
- Maternal diabetes screen
- Neurological assessment and thrombophilia screen of the neonate
- Treat underlying cause where possible
|
| Maternal vascular malperfusion |
- Maternal blood pressure, weight, glucose, liver and
renal function assessments
- Consider aspirin, specialist obstetric care, early delivery
- Long-term maternal cardiovascular disease risk assessment
- Specialist obstetric care with early delivery in future pregnancy if indicated
|
| Delayed villous maturation |
- Maternal diabetes screen and review of previous tests
- Advice to avoid excessive weight gain in future pregnancy
- Fetal movements monitoring in third trimester
- Specialist obstetric care with early delivery in future pregnancy
if indicated
|
Other hurdles to a full understanding of the clinical significance of placental pathology in obstetric care include the lack of standardisation in nomenclature and diagnostic criteria as well as interobserver variability among pathologists.1 To address these issues, a panel of international expert placental pathologists formulated the 2014 Amsterdam classification, terminology and definitions of placental lesions.5 Table 2 is a comprehensive list of placental pathology entities incorporating the new Amsterdam Placental Workshop Group terminology. This consensus nosology is expected to accelerate research on the understanding of the clinical implications of placental lesions in obstetric and neonatal care.
| Table 2. Classification of placental clinicopathological disorders incorporating 2014 Amsterdam Working Group nosology |
| Disease category |
Placental entity |
Infectious conditions of placenta
|
- Acute:
- Maternal inflammatory response: (sub)chorionitis, chorioamnionitis
- Fetal inflammatory response: funisitis, chorionic vasculitis or umbilical vasculitis
- Chronic:
- Villitis (syphilis, toxoplasmosis, rubella, cytomegalovirus, herpes simplex [‘STORCH’]; other)
- Histiocytic intervillositis (protozoal, malaria, other)
|
| Vascular conditions of placenta |
- Maternal vascular malperfusion:
- Distal villous hypoplasia/accelerated villous maturation
- Decidual arteriopathy
- Villous infarction
- Infarction haematoma/intervillous haemorrhage
- Retroplacental haemorrhage
- Fetal vascular malperfusion:
- Fetal vascular thrombosis
- Avascular sclerotic villi
- Delayed villous maturation
|
| Immune-mediated/idiopathic inflammatory conditions |
- Villitis of unknown aetiology
- Chronic deciduitis
- Chronic chorioamnionitis
- Chronic (histiocytic) intervillositis
- Eosinophilic T-cell fetal vasculitis
|
| Other placental disorders |
- Massive perivillous fibrin deposition (‘maternal floor infarction’)
- Chorangiosis
- Chorangiomatosis
- Placenta accreta, increta and percreta
- Umbilical cord abnormalities
- Macroscopic anomalies of placental disc and membranes
|
| Placental tumour-like lesions and neoplasms |
- Chorangioma
- Placental pseudocyst (extravillous trophoblastic cyst)
- Intraplacental choriocarcinoma
- Placental site trophoblastic tumour
- Other (eg ALK-rearranged inflammatory myofibroblastic tumour)
- Metastases – maternal origin
- Metastases – fetal origin
|
| ALK, anaplastic lymphoma kinase |
This review provides medical practitioners with an update on new terminology and status quo of research on placental entities of clinical significance that may recur in subsequent pregnancy and for which alteration of obstetric care may improve outcomes. With the advent of the electronic My Health Record system implemented Australia-wide, patients will have direct access to their histopathological reports online. As a result, it is likely that general practitioners will find themselves explaining the clinical significance of placental pathology reports to their patients more frequently than before.
This article focuses on the clinical significance of recurrent placental entities diagnosable on microscopic examination. Readers are referred to the aforementioned Amsterdam Workshop Group article5 for definitions and histological descriptions of these lesions. Neoplasms of the placenta are extremely rare, often sporadic and non-recurrent and are not discussed in this article.
Ascending intrauterine infection
Ascending intrauterine infection or amniotic fluid infection (AFI) sequence complicates up to 50% of preterm deliveries and is seen in 5–10% of term placentas. Acute chorioamnionitis or acute subchorionitis imply a maternal inflammatory response to ascending infection in the genital tract that has reached the amniotic sac (Figure 1A). Conversely, acute funisitis, chorionic vasculitis and umbilical vasculitis implies a fetal response to ascending intrauterine infection. The recommended histopathological grading of AFI is binary (grade 1 = mild, and grade 2 = severe), and the staging is three-tiered: stage 1 (early), 2 (intermediate) and 3 (advanced) for both maternal and fetal inflammatory responses.6
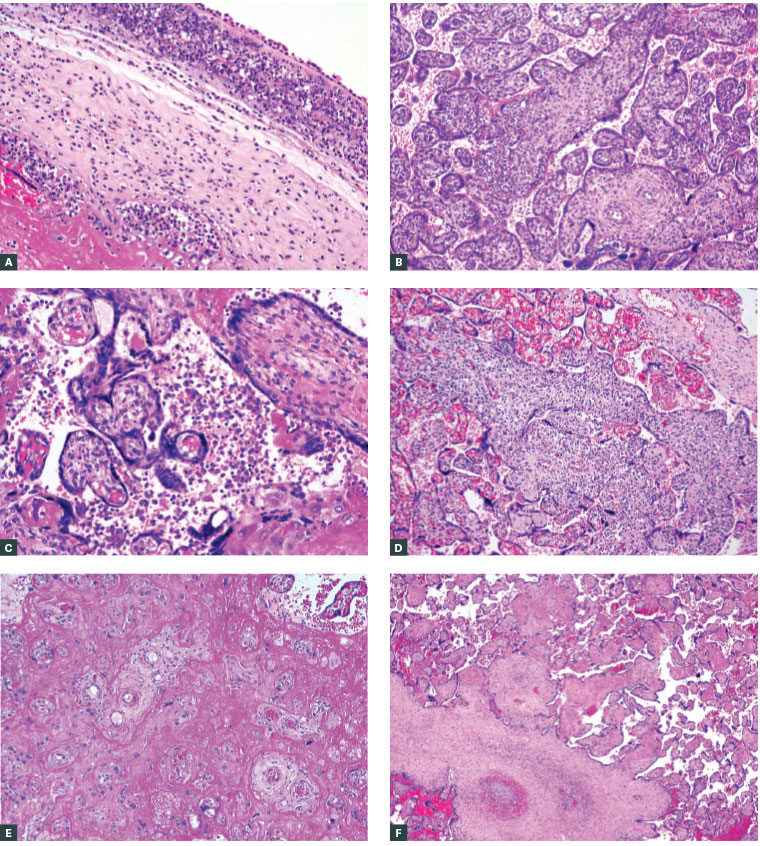
Figure 1. Inflammatory and other lesions of the placenta
A. Stage 3 acute chorioamnionitis with early necrotising inflammation of the amniotic basement membrane; B. Acute villitis in a case of fetal demise due to in utero Listeria monocytogenes infection; C. Chronic villitis of unknown aetiology involving terminal villi in the centre at low power; note the reduced vasculature, broad outlines and hypercellularity; D. Chronic histiocytic intervillositis (CHIV): histiocytic and lymphocytic cells aggregate between uninvolved chorionic villi. Malarial infection and idiopathic CHIV elicit similar histopathological features; E. Massive perivillous fibrin deposition; pink fibrinoid material encases the majority of chorionic villi in the placenta, rendering them non-functional for nutrient supply and oxygenation; F. Segmental fetal vascular malperfusion; occlusive thrombosis of stem villous vasculature associated with fibrotic and avascular distal terminal villi
Studies show some correlation between histopathological stage and duration of clinical AFI, while histopathological grade loosely correlates with clinical severity. Grade 1 AFI is often subclinical, while grade 2 lesions are more likely to be clinically apparent, with higher risk of perinatal morbidity and mortality.6 Stages 2 and 3 AFI with fetal inflammatory response significantly correlate with perinatal morbidity and are associated with increased likelihood of various neonatal conditions including culture-positive sepsis, respiratory distress and neurological impairment.7
Some bacteria and fungi elicit characteristic patterns of inflammatory response that may direct the pathologist to identify for the specific organism involved. For example, Listeria spp. infection often causes acute villitis (Figure 1B) in addition to high-grade acute chorioamnionitis, while acute funisitis due to Candida spp. is typically focal, necrotising and peripherally located. Candidal hyphae can be highlighted with fungal stains by the histopathologist; this is considered a critical result to be conveyed urgently to the treating team so that antifungal therapy may be prescribed in lieu of the empirical standard triple-antibiotic therapy.8
Chronic inflammatory conditions of the placenta
A histopathological diagnosis of chronic villitis (Figure 1C) requires the clinician to exclude viral and protozoal (eg cytomegalovirus, rubella, toxoplasmosis) infections in both mother and fetus. Fewer than 5% of cases of chronic villitis are due to infections in the developed world.9 In the majority of cases, no infection is found; this inflammation is termed villitis of unknown aetiology (VUE). A binary grading system for chronic villitis and deciduitis is also in use.5 Low-grade chronic villitis, after exclusion of infections, does not have a known clinical significance. Conversely, high-grade VUE shows consistent association with adverse pregnancy outcomes including fetal growth restriction, recurrent pregnancy loss and intrauterine fetal death.9,10 Severe VUE may be accompanied by thrombosis in fetal vasculature, in which case the risks of neurological deficits in the neonatal period and long-term cerebral palsy are high. Studies show recurrence rates of VUE of up to 37% in subsequent pregnancy for some individuals.9,10
Evidence on how best to manage VUE in subsequent pregnancy is lacking because of a dearth of randomised control studies comparing potential treatment options suggested from case reports and laboratory studies. Anecdotal and limited experimental evidence from one French group suggest that use of aspirin 100 mg/d combined with prednisolone 20 mg/d from early second trimester may reduce the recurrence rate of VUE and possibly improve outcomes in future pregnancy for affected individuals.11 Weekly maternal intravenous immunoglobulin (1 g/kg) from late first trimester significantly reduced the occurrence of VUE associated with neonatal alloimmune thrombocytopaenia (NAIT) in one small study.12
Chronic inflammation involving other placental structures – such as the decidua (chronic deciduitis), fetal membranes (chronic chorioamnionitis), intervillous space (chronic histiocytic intervillositis [Figure 1D]) and fetal vessels (eosinophilic T-cell vasculitis) – can occur either in isolation or accompanying high-grade VUE and is also thought to have an immune-mediated pathogenesis.
For all forms of high-grade chronic inflammatory conditions of the placenta, expert recommendations for subsequent management include a maternal thrombophilia and autoimmunity screen, neonatal screen for NAIT and neurological deficits, review of the clinical history for a possible viral or protozoal cause, and review of any previous placentas and products of conception for recurrent chronic inflammation.3,4 Specialist antenatal care with intensive monitoring and elective early delivery are also recommended for future pregnancy.
Massive perivillous fibrin deposition and maternal floor infarction
Massive perivillous fibrin deposition (MPFVD) and the associated entity maternal floor infarction (Figure 1E) are rare, complicating up to 0.5% of pregnancies. They are clinically associated with otherwise unexplained fetal growth restriction and, sometimes, fetal demise.13 Recurrence rates for these lesions are very high, with rates of up to 89% reported in some series.14 Clinical management implications, including empirical treatment of recurrent MPFVD, are the same as for VUE. Pravastatin has also been used successfully in one patient with recurrent MPFVD.15
Fetal vascular malperfusion
Fetal vascular malperfusion (FVM) is the currently preferred term for lesions previously called fetal thrombotic vasculopathy. It affects 4–6% of placentas sent to pathologists for examination.16 The syndrome encompasses the following histopathological lesions and terms: avascular villi (global and segmental), occlusive and non-occlusive thrombosis in fetal vasculature (Figure 1F), vascular intramural fibrin deposition, endothelial cushions, vascular ectasia, haemorrhagic endovasculitis, villous stromal-vascular karyorrhexis, fibrinous vasculosis, thrombosclerosing placentitis, endarteritis obliterans and fibromuscular sclerosis.17 Risk factors for FVM include gestational diabetes, hypercoiled umbilical cord and cord accident.
A grading system for FVM has been proposed.5 Low-grade FVM may not have clinical significance, whereas high-grade FVM shows a strong correlation with neurological impairment in the fetus or newborn, neonatal stroke, seizures and long-term neurological deficits.18 Severe FVM is also associated with a higher likelihood of perinatal death.
Suggested clinical management of an FVM diagnosis includes neurological assessment and thrombophilia work-up of the neonate, together with diabetes screening for the mother.4 A placental diagnosis of FVM may help account for otherwise unexplained abnormal Doppler ultrasonography findings, fetal distress, a neurologically impaired neonate that requires intensive care admission and perinatal death. Treatment of, and monitoring for, any underlying cause, where present, may prevent recurrences in subsequent pregnancies.
Maternal vascular malperfusion
Maternal vascular malperfusion (MVM) is the currently recommended umbrella term for pathological placental findings that are manifestations of chronic under- or overperfusion due to any cause. Histopathological lesions of MVM include small placental disc (<10th percentile for gestational age), pathological villous infarction (all pre-term infarcts [Figures 2A and 2B], multiple infarcts at any gestational age, and term infarcts >5% by volume; a small isolated peripheral focus at term is excluded), infarction haematoma, distal villous hypoplasia (Figure 2C), accelerated villous maturation, decidual vasculopathy (Figure 2D) and laminar decidual necrosis.5
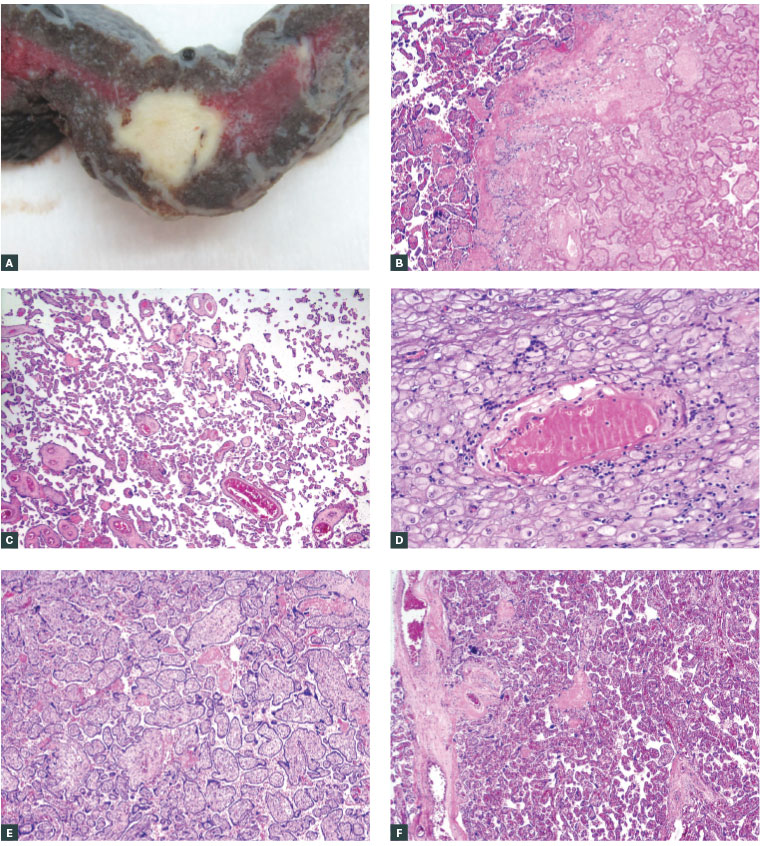
Figure 2. Maternal vascular malperfusion
A. Cut surface of a 32 weeks’ gestation placenta showing an off-white solid focus of villous infarction; B. Corresponding microscopy of the edge of the villous infarct shown in Figure 2A, showing ghost outlines of villi that are clumped together into a solid mass. Chorionic villi to the left of the infarct show features of accelerated villous maturation for 32 weeks; C. Distal villous hypoplasia at 35 weeks’ gestation: chorionic villi are smaller than normal, widely spaced with slender outlines and prominent, wavy syncytiotrophoblasts; D. Acute atherosis in a spiral artery of the uterine decidua. There is intramural fibrinoid necrosis with foamy histiocytic aggregates and surrounding lymphocytes; E. Delayed villous maturation in a 38 weeks’ gestation pregnancy complicated by otherwise unexplained sudden intrauterine fetal demise at term. Villous maturation resembles that seen in early second trimester. Villi are broad with increased stroma and reduced vasculo-syncytial membranes; F. Normal villous maturation at term for comparison
MVM lesions are caused by maternal systemic conditions that compromise blood flow to the placenta and fetus. Hypertensive disorders (pre-eclampsia; eclampsia; chronic hypertension; hemolysis, elevated liver enzymes, low platelet count [HELLP] syndrome) are the most common and most studied.19 Acute atherosis – the constellation of intramural fibrinoid deposition in decidual vessels with foamy histiocytic and perivascular lymphoplasmacytic infiltration – is almost pathognomonic for severe pre-eclampsia complicated by intrauterine growth restriction (Figure 2D). An increased risk of maternal cardiovascular disease later in life after acute atherosis has recently been highlighted.20 Maternal diabetes and obesity,21 antiphospholipid syndrome22 and smoking23 have been shown to be associated with variable combinations of these lesions, the extent of which likely depends on the severity of disease and antenatal treatment administered. Thus, a finding of MVM lesions in a placenta is a surrogate for evidence of the impact of systemic maternal conditions and may be useful in assessing effectiveness of antenatal management as well as how to better manage affected individuals in subsequent pregnancies. Severe MVM is a risk factor for perinatal death, neonatal bronchopulmonary dysplasia24 and neurological impairment in the neonate.25,26
Delayed villous maturation
Delayed villous maturation (DVM; ‘villous dysmaturity’) occurs in 2–5% of pregnancies and is most often seen in pregnancies complicated by gestational diabetes, hypercoiled cord, obesity, and excessive weight gain during pregnancy.27 An idiopathic form of DVM (Figure 2E) occurs in the absence of known gestational or chronic diabetes, and recent research suggests abnormal growth factor expression or an underlying delay in gene expression to be potential causes.28
The most common setting of idiopathic DVM in clinical practice is an initially unremarkable pregnancy, with normal growth parameters during the first and second trimesters, that is complicated by sudden and unexplained fetal distress or perinatal death at or near term (>35 weeks). Autopsy often reveals a normally grown fetus with no congenital anomaly and a large placenta with DVM features. Idiopathic DVM causes intrauterine fetal demise in up to 2% of pregnancies, and its recurrence rate is approximately 10%.27
Recommendations for clinical management of subsequent pregnancies in the setting of idiopathic DVM complicated by fetal distress or perinatal death includes a diabetes screen, avoidance of excessive weight gain, third trimester fetal movement monitoring and earlier delivery before full term.4
Conclusion
A lack of universally accepted diagnostic criteria, terminology and grading systems has hindered the full potential of placental histopathology in fetomaternal care.1 The universal adoption of the Amsterdam criteria by reporting pathologists and obstetricians is a positive and welcome step in developing uniformity of nomenclature. More research efforts and resources should be directed towards studies to elucidate further what is currently unknown: definitive pathogenetic mechanisms, local epidemiology, exact impact on perinatal morbidity and mortality, evidence-based management guidelines and therapy for aforementioned recurrent idiopathic placental conditions.
Key points
- When reported adequately and interpreted correctly, placental histopathology provides useful information that may explain adverse outcomes and guide further management of the mother, newborn or future pregnancy.
- Recurrent placental conditions such as VUE, chronic histiocytic intervillositis, vascular malperfusion, MPFVD and DVM are associated with an increased risk of perinatal morbidity and mortality.
- Empirical treatment of these conditions with prednisolone, aspirin, low molecular weight heparin, hydroxychloroquine and intravenous immunoglobulins are documented. However, evidence-based guidelines for the management of these conditions are lacking.
- More research efforts and resources should be directed towards studies to elucidate further what is currently unknown: definitive pathogenetic mechanisms, local epidemiology, exact impact on perinatal morbidity and mortality, evidence-based management guidelines and therapy for recurrent idiopathic placental conditions.