Acute sore throat is a common reason for presenting to primary healthcare.1 Although most cases of a sore throat are viral in origin, a sore throat caused by group A β-haemolytic streptococci (GAS) can occasionally have suppurative complications as well as significant non-suppurative sequelae such as rheumatic fever and glomerulonephritis. The risk for rheumatic fever is very low in most high-income countries but may be higher in low-income countries.2 Australia and New Zealand are high-income countries that incur a high incidence of rheumatic fever, especially among people living remotely and Aboriginal and Torres Strait Islander peoples in Australia and Maori peoples in New Zealand.3,4
Aetiology of the sore throat
The aetiology of the majority of sore throats is a viral infection, but a clinically relevant number of cases are caused by GAS. Other bacterial aetiologies potentially involved are Streptococcus dysgalactiae subspecies Equisimilis (SDSE),5–7 Fusobacterium necrophorum8,9 or other bacteria.10 SDSE was previously labelled group C β-haemolytic streptococci or group G β-haemolytic streptococci. However, systematic reviews focusing on case–control studies have not established that SDSE is linked to the uncomplicated acute sore throat to the extent that it should be considered in the management of these patients.11–14
Antibiotic treatment of patients with an acute sore throat
Antibiotics reduce acute symptoms of sore throats in children,15–17 and in children and adults combined, if GAS is present.18–20 Although antibiotic treatment seems effective in primary prevention of rheumatic fever,3,21,22 the magnitude of the effect on acute symptoms of an uncomplicated acute sore throat is modest.23 Most current studies show no effect in children15,16 and children and adults combined18 if GAS is not present. The exceptions are one study by Petersen,24 focusing on GAS-negative patients, and a study by Zwart,25 focusing on patients with presence of SDSE and absence of GAS. Both showed a borderline effect on symptoms of an acute sore throat in adults with P values of 0.049 and 0.05, respectively. There are, to date, no studies of antibiotic treatment for an uncomplicated sore throat suspected to be caused by F. necrophorum.
All studies that compared the effect of antibiotics with placebo, and performed a separate analysis for GAS-positive and GAS-negative patients, found an effect in GAS-positive patients and none in those who were GAS-negative.15,16,18,20 These studies show there is firm evidence in children and adults for a modest effect of antibiotics on the acute symptoms of acute sore throat if GAS is present. There is only very weak support for an effect in adults if GAS is not present, and no evidence for children with no proven growth of GAS. Furthermore, the reduction in risk for rheumatic fever and the modest reduction in acute symptoms proven in previous intervention studies must be weighed against potential side effects of antibiotic treatment.
Symptomatic carriers and testing presence of GAS
GAS may sometimes be present in healthy individuals, who are labelled ‘asymptomatic carriers of GAS’. An incorrect conclusion about the aetiology of symptoms might be drawn if these asymptomatic carriers of GAS acquire a viral pharyngitis and become symptomatic carriers of GAS.26, 27 Patients attending for a sore throat are a mixture of those ill from a virus, symptomatic carriers of GAS, those ill from GAS and those ill from other bacteria.26,27 Tests for presence of GAS cannot distinguish between symptomatic carriers of GAS and those ill from GAS.
The negative predictive value of a test to detect GAS, while also considering symptomatic carriers, is always very high (97–99%).26,28,29 Most tests for presence of GAS will be negative in this mixture of patients and this is very effective at ruling out GAS as the cause of the sore throat, even in the presence of symptomatic carriers ill from something other than GAS.26,28,29 Hence, a negative point-of-care test (POCT) can be used to stop an intended antibiotic prescribing in uncomplicated cases of acute sore throat. In most scenarios, this is the main value of a POCT to detect GAS.29
The clinical value of a positive test for GAS varies depending on the proportion of symptomatic carriers of GAS.26,28 In most scenarios, a positive test for GAS in a patient with a sore throat has a high probability of indicating the aetiology.28
Clinical evaluation and point-of-care testing
Many different clinical scoring algorithms can be used to try to identify patients for whom antibiotic treatment may be beneficial. The most widely used is the Centor criteria.30 All clinical scoring algorithms, including the Centor criteria, have inherent low sensitivity and specificity.31 Relying solely on the Centor criteria means many patients ill from GAS are left without antibiotics while many patients ill from a virus are prescribed antibiotics.32
Given the current evidence for the effect of antibiotic treatment in patients with an uncomplicated acute sore throat, it seems reasonable to emphasise identifying the presence of GAS before an antibiotic prescription.29 A throat swab sent for culture is of limited value for the decision to prescribe antibiotics because of the long delay before the result is available.33 However, analysing the throat swab with a high-quality rapid POCT, delivering a result while the patient is still at the premises, might be very useful.28,29,32 Modern POCT for GAS have a sensitivity and specificity to detect GAS on par with, or even better than, conventional culture techniques.34
The objective of the present study was to investigate to what extent the introduction of a high-sensitive polymerase chain reaction (PCR) POCT to detect presence of GAS changes the management of otherwise healthy patients attending for an uncomplicated acute sore throat.
Methods
This prospective study was approved 5 March 2018 by the Townsville Hospital and Health Service Human Research Ethics Committee (registration number HREC/17/QTHS/246).
Study design
Patients at three sites in North and North-West Queensland, Australia, attending for a sore throat, and their treating medical practitioner, were asked to participate in a prospective observational study.
Study objectives
The study aimed to clarify to what extent medical practitioners’ decisions to prescribe antibiotics are changed by a POCT. The study also aimed to explore factors associated with prescribing antibiotics despite knowing a PCR test indicates no presence of GAS.
Practitioners, patients and recruitment
All medical practitioners at the three sites managing patients with an acute uncomplicated sore throat were invited to participate. Practitioners were asked to consider including all consecutive patients attending Mt Isa Base Hospital emergency department, Hinchinbrook Health Care in Ingham or One Stop Medical in Mackay who presented with a main complaint of an acute sore throat, unless it was a representation for the same illness episode or if the medical practitioner deemed the patient so ill that admission to hospital was required. Hinchinbrook Health Care and One Stop Medical are primary healthcare centres. The emergency department in Mt Isa typically manages acute emergency department patients, but also lower acuity problems, including patients presenting with a sore throat.
Data collection
Data were collected on a case report form (CRF) based on a CRF that worked well in a previous study.28,32 On the first page of the CRF, information was retrieved about the treating medical practitioner’s level of education and patient’s age, sex, cultural identity, symptoms and signs, and the medical practitioner’s clinical diagnosis based on symptoms and signs. Retrieved information made it possible to provide a Centor score on each patient as a pre-test probability of the patient having GAS present. The Centor score consists of four clinical signs or symptoms, each adding to a total Centor score from zero to four: tonsillar exudates, swollen tender anterior cervical nodes, lack of a cough and a history of fever.30 Finally, the medical practitioner stated if they would prescribe antibiotics on the basis of the clinical assessment.
A throat swab for PCR POCT was performed after the first page of the CRF was completed and the outcome was registered at the top of page two. Swabs were taken either by the medical practitioner or a trained nurse. Medical practitioners, and in some sites nurses, were already taking throat swabs and had long experience of doing this. In addition, they were also informed in writing about the best technique to obtain an optimal throat swab sample. Nurses were employed by the clinic. After obtaining the test result, the CRF required the medical practitioner to note on page two if they would prescribe antibiotics knowing the test outcome. The medical practitioner was also asked about the main reason for prescribing antibiotics despite a negative test (if that was the case).
Any antibiotic prescription was classified by the medical practitioner as for immediate consumption or delayed consumption, meaning the patient was instructed to start medication later if symptoms remained or became worse. The exact recommended delay may have varied between practitioners and this was not registered.
The CRF had no code or signature linking the information to a particular patient or treating medical practitioner. Hence, the information collected on the CRF was anonymous.
Test for presence of GAS
Before this study, neither Hinchinbrook Health Care nor One Stop Medical had used a POCT in managing patients with a sore throat. The Mt Isa Hospital emergency department had previously used a rapid antigen detection test as a POCT for children attending with a sore throat when they participated in a study during the period June 2014 to February 2015.28,32
All patients were swabbed and the presence of GAS was analysed using Abbott ID NOW Strep A (formerly Alere i Strep). This is a nucleic acid test using LAMP technology that can be described as an isothermal PCR with similar test characteristics as conventional PCR. This test has a >95% sensitivity and >95% specificity to detect GAS when compared with in-house PCR35 or culture.36 Participating medical practitioners were informed about the characteristics of this test. Nurses at each clinic received a one-hour training session from Abbott (formerly Alere) to run the test. Nurses were employed by the clinic. The test took approximately eight minutes to analyse, after which time the patient returned to the medical practitioner. This test was at the time of the study approved by the Australian Therapeutic Goods Administration for use in patients with a sore throat.
Statistical analysis
Antibiotic prescribing patterns before and after the outcome of the PCR test for GAS were presented with descriptive statistics. Sites were compared using two-sided chi-square or t-test (Appendices, available online). Data for antibiotics for immediate or delayed consumption were merged and chi-square was used to test the hypothesis that the POCT resulted in a changed prescribing behaviour. A multivariable logistic regression was used to clarify if the medical practitioner’s level of training, patient’s age, patient’s sex, patient’s cultural identity or the Centor scores were associated with an increased risk of prescribing antibiotics despite proven absence of GAS defined as a negative test outcome of the PCR test.
Sample size calculation
A sample size calculation was made for the potential difference in antibiotic prescribing in case of a negative test for GAS. It was assumed that 20% of general practice trainees (registrars) would prescribe antibiotics despite a negative test for GAS, in comparison to 40% for specialist general practitioners, assuming a level of significance of 0.05, a power of 0.8 and a two-sided test requires 207 patients. The software G*Power version 3.1.9.2 was used assuming logistic regression with antibiotic prescribing as the dependent variable.37,38 The researchers aimed to collect data from 300 patients.
Role of the funding source
This study was funded by James Cook University and Bond University, both in Australia. Some of the authors are employed by these universities. The PCR devices and test kits were purchased from Abbott. Hence, Abbott was not a funder and had no influence on study design, analysis of data or writing of the manuscript.
Patient and public involvement
Neither patients nor the public were involved in the design of this study.
Results
Two hundred and eighty-three patients were included from April 2018 to February 2019. Recruitment ceased as the available test kits expired. Half of the patients were between 11 and 36 years with a slight predominance of females (Table 1; Appendix 1, available online only). The Mount Isa site had, as expected, a larger proportion of Aboriginal and Torres Strait Islander patients (Appendix 1). The PCR test showed presence of GAS in 37% (27/57) of Aboriginal and Torres Strait Islander patients, while it was only positive in 25% (50/204) of Caucasian patients (P = 0.00082, chi-square). There was no sex difference in the proportion of positive tests for presence of GAS (P = 0.35, chi-square).
| Table 1. Demographic information, symptoms, presence of group A streptococci and treating practitioner for included patients (n = 283) |
| Characteristic |
% (n)* |
| Female sex (n = 283) |
62 (176) |
| Aboriginal or Torres Strait Islander (n = 281) |
21 (59) |
| Age in years (n = 282) |
| Mean (standard deviation) |
25 (19) |
| Median (interquartile range) |
22 (11–36) |
| Min–max |
0–96 |
| Symptoms and signs |
| Absence of cough (n = 283) |
42 (119) |
| Tonsillar exudate (n = 283) |
24 (69) |
| Tender anterior cervical lymph nodes (n = 283) |
37 (104) |
| Fever >38 °C (n = 283) |
36 (103) |
| Centor score registered (n = 283) |
| Centor score 0 |
23 (65) |
| Centor score 1 |
34 (95) |
| Centor score 2 |
28 (78) |
| Centor score 3 |
13 (36) |
| Centor score 4 |
3.2 (9) |
| Presence of group A streptococci in the throat (n = 277) |
31 (85) |
| Educational level among medical practitioner for each consultation (n = 281) |
| General practitioner/senior medical officer |
33 (92) |
| Junior practitioner under training (registrar/principal house officer) |
59 (166) |
| Neither of above |
8.2 (23) |
| *Percentage and number are shown for all characteristics except age |
Pre-test prescribing intention
Before the PCR POCT, almost all patients perceived to have a bacterial infection were prescribed antibiotics (Figure 1). This included patients with 0–2 Centor scores, of whom 37% were prescribed antibiotics (Figure 1). Antibiotics were prescribed to 52% (31/59) of Aboriginal and Torres Strait Islander patients and to 44% (92/208) of Caucasian patients (P = 0.26, chi-square).
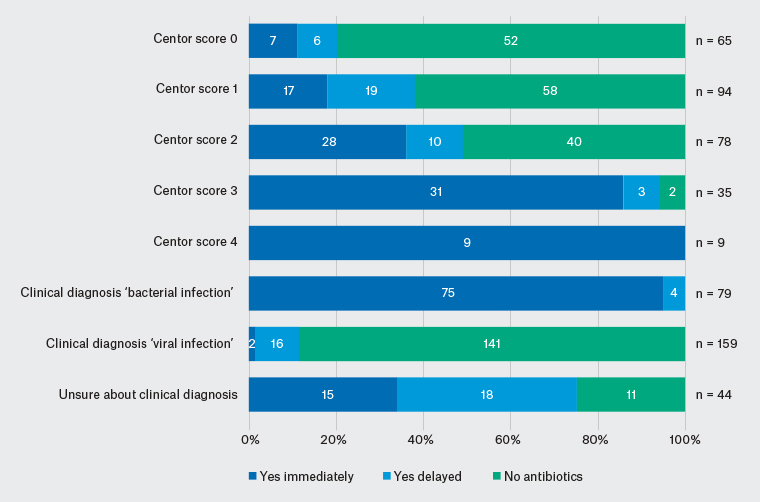
Figure 1. Antibiotic prescribing pattern before result of polymerase chain reaction point-of-case test for group A streptococci was available
Change incurred by introducing the PCR test
The outcome of the PCR test as well as the pre-test and post-test antibiotic prescribing was recorded for 276 patients (Table 2; Appendix 2, available online only). Patients were prescribed antibiotics both pre- and post-test in 81/276 (29%) of cases while 112/276 (41%) were not prescribed antibiotics pre- or post-test. However, the decision regarding antibiotic prescribing was changed for 83/276 (30%) of patients (P <0.001, chi-square; Table 2). Hence, introducing the PCR POCT only reduced the proportion of patients prescribed antibiotics from 46% to 40%; but the management was actually changed for 30% of patients. The proportion of patients prescribed antibiotics that most likely had GAS in their throats increased from 51/128 (40%) to 114/117 (97%; Table 2). Furthermore, those patients with a sore throat with GAS in their throat and not being prescribed antibiotics dropped from 40% (34/85) pre-test to 1.2% (1/85) post-test.
| Table 2. Change of antibiotic prescribing pattern after result of test for group A streptococci was available |
| Pre-test antibiotic prescribing |
PCR test for GAS |
Post-test antibiotic prescribing* |
| |
|
No |
YD |
YI |
n |
| No |
Negative |
111 |
2 |
1 |
114 |
| Positive |
1 |
0 |
33 |
34 |
| YD |
Negative |
23 |
4 |
1 |
28 |
| Positive |
0 |
1 |
9 |
10 |
| YI |
Negative |
24 |
2 |
23 |
49 |
| Positive |
0 |
0 |
41 |
41 |
*Changes from pre- to post-test are marked bold
GAS, group A streptococci; No, no antibiotics; PCR, polymerase chain reaction; YD, antibiotics prescribed delayed; YI, antibiotics prescribed for immediate consumption |
Antibiotic prescribing in case of a negative test for presence of GAS
Testing for GAS was negative in 69% (192/277) of patients. In 77/192 patients this was unexpected and the practitioner pre-test had intended to prescribe antibiotics either immediately (n = 49) or delayed (n = 28). The treating medical practitioners still decided to prescribe antibiotics for 30 of these 77 patients, despite a negative PCR POCT. The reasons stated for this were: that the medical practitioner suspected the patient had a throat infection caused by a bacteria other than GAS (n = 17), that the patient was ill from GAS despite a negative PCR test (n = 1), request from the patient (n = 1), throat swab was difficult to obtain (n = 1), patient was diagnosed with serous otitis media/glue ear (n = 1), patient was diagnosed with ‘chest infection’ (n = 1) and no reason was stated for eight patients.
Multivariable logistic regression (n = 190) revealed that an increase in Centor score by one increased the chance of antibiotic prescribing, despite a negative PCR test for presence of GAS, with an odds ratio of 2.2 (95% confidence interval [CI]: 1.4, 3,5; P = 0.00091; Table 3). Furthermore, specialist general practitioners were more prone to prescribe antibiotics despite a negative PCR test for GAS, compared with general practice trainees (registrars), with an odds ratio of 4.8 (95% CI: 1.8, 12; P = 0.0013; Table 3). No other variables were associated with antibiotic prescribing despite a negative test for GAS.
| Table 3. Factors associated with antibiotic prescribing in patients with a sore throat and no presence of group A streptococci (n = 190) |
| |
Adjusted odds ratio
(95% confidence interval)
|
P value |
| Level of training of medical practitioner |
| General practitioner (GP)/senior medical officer (SMO) |
4.8 (1.8, 12) |
0.0013 |
| Neither GP/SMO nor registrar |
1.2 (0.12, 12) |
0.87 |
| Registrar under education |
(Reference) |
0.0051 |
| Increase of patient’s age (one year) |
0.99 (0.97, 1.0) |
0.55 |
| Female sex of the patient |
0.85 (0.35, 2.0) |
0.71 |
| Cultural identity |
| Aboriginal or Torres Strait Islander |
2.2 (0.17, 29) |
0.54 |
| Caucasian |
1.3 (0.13, 13) |
0.83 |
| Other |
(Reference) |
0.69 |
| Increase of Centor score one step |
2.2 (1.4, 3.5) |
0.00091 |
Antibiotic prescribing in case of an unexpected positive test for presence of GAS
The test for GAS was unexpectedly positive among 34 patients where the practitioner pre-test had chosen to not prescribe antibiotics. This resulted in an antibiotic prescription in 33 of these 34 patients (Table 2).
Discussion
This study found that introducing a PCR POCT test helped practitioners direct antibiotic prescribing to patients with a sore throat and GAS in their throats. This meant that many patients not prescribed antibiotics pre-test were prescribed antibiotics post-test and vice versa. Overall, the management was changed for 30% of patients, with a shift from prescribing antibiotics to patients without GAS to those with GAS. The overall reduction in patients prescribed antibiotics was modest, but antibiotics were better targeted to patients likely to be ill from GAS. Hence, the overall value of POCT testing would be from the improved targeting of antibiotic prescriptions, not the overall reduction.
A substantial proportion of patients with 0–2 Centor criteria were prescribed antibiotics both pre- and post-testing for presence of GAS. A negative PCR often resulted in an antibiotic prescription and an unexpected positive PCR test almost always resulted in an antibiotic prescription. Senior medical practitioners were much more likely to prescribe antibiotics to patients with no presence of GAS than junior practitioners, perhaps because junior practitioners have encountered POCT during their recent training, making them trust the POCT more, or they may have a more recent education in antibiotic stewardship. All these findings should trigger a healthy discussion about the need for a changed behaviour among medical practitioners.
Strength and limitations
This study did not only investigate the total reduction in antibiotic prescribing, which was modest, but also the change towards more targeted antibiotic prescribing, which was large. This is highly relevant for patients at high risk for developing rheumatic fever. The rate of positive tests for GAS was higher in this study (31%), than in many other studies of patients with a sore throat in primary care. The total reduction in antibiotic prescribing would probably be more pronounced in settings with a lower prevalence of GAS.
A potential limitation is that recruitment was stopped before the target of 300 patients was achieved. However, the study managed to recruit well over the 207 patients estimated by the sample size calculation and it was deemed unnecessary to apply for further funding to purchase more test kits.
Information retrieved on the CRF was anonymous, and one consequence is that the exact number of medical practitioners participating in the study in unknown. Another potential limitation is that medical practitioners may have ignored the study in times of high patient load and not all consecutive suitable patients are likely to have been invited. However, the allocation between Centor scores in Table 1 is similar to what is seen in other recent studies with few patients fulfilling all four Centor criteria,32,39 suggesting that this problem is unlikely to have introduced a systematic error.
Finally, the less widely used but more recent McIsaac scores were not used in this study. The difference between the Centor and McIsaac scores to select patients at high probability for a GAS sore throat is marginal.40 The results reflect short-term intended behaviours, and it is not known how this may change with continued usage.
Presence of GAS and POCT
Guidelines suggesting antibiotic treatment on clinical grounds irrespective of presence of GAS or SDSE7 makes POCT irrelevant. The consequence is an encouragement to rely on clinical symptoms and signs rather than the more objective throat swab. This behaviour significantly increases antibiotic prescribing29,32 and leaves a significant proportion of patients ill from GAS, even in areas with a high incidence of rheumatic fever, without antibiotics,32 which is not acceptable. The latter is emphasised by the fact that testing reduced the proportion of patients with GAS not prescribed antibiotics from 40% to 1.2%.
Availability of POCT
The cost of POCT includes costs for maintaining approval with regulatory authorities and balancing stocking an adequate number of test kits with wastage due to expiry dates. Countries such as Australia, where POCT is not recommended in primary care guidelines, are likely to be considered a dead market and the registration may be withdrawn. Hence, the recommendations suggested above are unlikely to be successful in Australia unless the therapeutic guideline for management of patients with a sore throat41 is updated to include POCT.
The requirement for accreditation with a continuous quality assurance process is a limiting factor. This would be simple to set up if an organisation would take responsibility for arranging dummy samples that could be sent to clinics for analysis. This is currently unavailable in Australia. Another limiting factor is that the overall cost for equipment, test kits, accreditation and staff time does not match the current reimbursement from the Medicare Benefits Schedule (MBS). It is likely that POCTs providing early accurate diagnosis and subsequent treatment would result in better outcomes, fewer hospital admissions and fewer lost days of productivity and this would be an argument for introducing an adequate MBS reimbursement for such tests. Although, this should be confirmed in a health economic analysis.
Conclusions and implications for general practice
Introducing a POCT to detect GAS made antibiotic prescribing much more targeted from prescribing to patients with no GAS present to those having GAS. Consequently, the POCT significantly reduced the risk for patients with GAS being left without antibiotics; this is especially important when the risk for rheumatic fever must be considered. The most-used guideline in primary healthcare in Australia41 does not mention POCTs, so most practitioners in Australia are unfamiliar with them. As Tarca et al42 previously suggested, there is also a need for an evidence-based single Australian national sore throat guideline where the role of POCTs to detect GAS is clarified.
Appendices 1 and 2.