Paget’s disease of bone (PDB) is a benign disorder of bone characterised by focal areas of disorganised bone turnover in a single bone (monostotic PDB) or multiple bones (polyostotic PDB).1 It is the second most common metabolic bone disorder after osteoporosis, affecting 1–2% of adults over the age of 55 years.2,3
The disease is usually asymptomatic but can be associated with increased fracture risk, deformity, bone pain and complications such as deafness and osteosarcoma.4
The prevalence of PDB is highest in northwest England and in countries where there is prominent British ancestry, such as Australia and New Zealand. Epidemiological studies suggest that the prevalence and severity of PDB has been decreasing over recent decades.2,3 Although the cause of this reduction is not completely understood, environmental changes – such as improved diet, sedentary lifestyle and decrease in the exposure to viral infections and zoonoses – might play a part.3–5
Risk factors
While the aetiology of PDB is still unclear, PDB is strongly genetic, with 40% of affected individuals having a positive family history.4,5 First-degree relatives of patients with PDB have an approximately sevenfold greater risk for the development of Paget’s disease.5–7 The most common mutations associated with PDB occur in the sequestosome 1 (SQSTM1) gene, which occurs in 20–50% of familial PDB and in 5% of those without a family history.7,8 The SQSTM1 gene maps to chromosome 5q35 and encodes a scaffolding protein, ubiquitin, which is important in the growth and activation of osteoclasts. Alterations in the signalling of this protein appear to contribute to the pathogenesis of PDB.7
At least 13 other susceptibility genes have been identified on genome-wide association studies.9
Environmental risk factors for PDB include early life exposure to wood fire heating, viral infections such as measles, paramyxovirus and respiratory syncytial virus, and environmental toxins such as lead and cadmium.9–11 Incidence of PDB has decreased in countries that began using measles vaccination in the 1960s.12 An association between PDB and smoking and excessive mechanical loading of the skeleton has also been reported.10,11
Clinical presentation
In 70–90% of cases, PDB is asymptomatic and the diagnosis is made incidentally on radiological imaging. PBD most commonly affects the pelvis (58–80%), spine (40%), femur (32%) and tibia (16–20%).13,14 In cases that present clinically, bone pain is the most common symptom, affecting 73% of patients in a recent meta-analysis.14
Many of the clinical features and complications of PDB are related to the abnormal areas of bone remodelling. The affected bones are at risk of bending and fracture, while bone enlargement can cause changes in facial appearance, hearing loss, basilar invagination of the skull, obstructive hydrocephalus, nerve entrapment, spinal canal stenosis and paraplegia.15,16 The increased vascularity of bone can cause excess surgical bleeding if orthopaedic surgery is necessary and delayed union in the event of fracture.14–17
Pain is differentiated into primary pain (related to the increased activity and vascularity of the pagetic bone) and secondary pain (more common, and due to complications such as nerve entrapment, osteoarthritis or joint deformity).14,16 Primary pain is dull, deep pain that is predominantly nocturnal.17 There is a weak correlation between bone pain and metabolic bone activity reflected by total alkaline phosphatase (ALP) concentrations, where 40–50% of patients experience no pain despite high levels of ALP.17
Facial appearance can change due to enlargement of the skull and facial bones.15
Complications of PDB include arthropathy due to alterations of the subchondral bone, fractures, compression neuropathy due to bone growth, and neurological dysfunction related to vascular steal syndrome (Table 1).17,18
| Table 1. Complications and symptoms of Paget’s disease of bone4,13,14 |
| Organ system |
Complication (prevalence %) |
| Bone |
Bone pain (52%)
Bone deformity (22%)
Fracture (9%)
Osteosarcoma (0.3%) |
| Joint |
Osteoarthritis (73%) |
| Neurological |
Deafness (9%)
Nerve root compression (4%)
Peripheral nerve compression (2%)
Compression neuropathy (4%)
Basilar invagination (2%)
Cranial nerve palsies (0.4%)
Paraplegia, quadriplegia, vascular steal syndrome |
| Cardiac |
High-output congestive cardiac failure (3%)
Aortic stenosis
Generalised atherosclerosis
Endocardial calcification |
| Metabolic |
Hypercalcaemia (5%)
Hypercalciuria
Nephrolithiasis
Hyperuricaemia |
Pathology
The pathognomonic feature of PDB is the abnormally active osteoclasts, which are increased in size and number. These osteoclasts secrete enzymes that dissolve mineralised bone and matrix to form lytic lesions, and stimulate increased numbers of osteoblasts to form disorganised, highly vascular cancellous bone that is prone to fracture (Figure 1).19 Unlike the lamellar distribution of mature adult bone, pagetic bone is a mixture of abnormal woven bone, disorganised cement lines and increased volume of unmineralised osteoid. The marrow is sclerosed and hypervascular.1,6
In the early stages, pagetic bone appears as lytic lesions radiographically. As osteoblasts form new bone, the lesions then become progressively sclerosed and deformed.
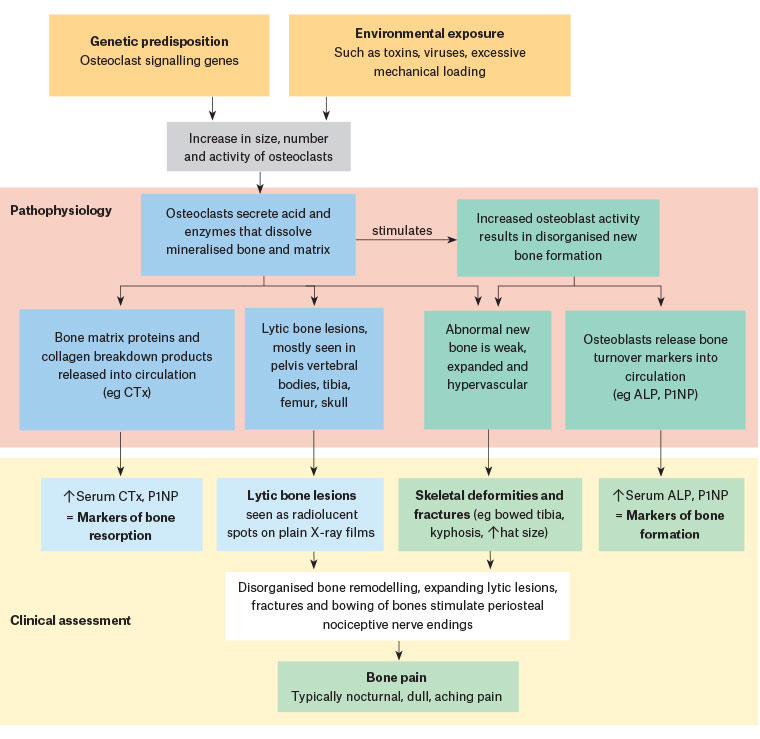
Figure 1. Pathogenesis and clinical presentation of Paget’s disease of bone19
ALP, alkaline phosphatase; CTx, C-terminal telopeptide pyridinoline crosslinks; P1NP, procollagen type 1 amino-terminal propeptide
Diagnosis
PDB is commonly asymptomatic, and the diagnosis is often made incidentally on the basis of an elevated ALP in the absence of liver disease or on radiological findings of PDB when imaging is being performed for other medical problems.6
Biochemical markers
Initial biochemical evaluation should be done using serum total ALP, which is elevated in untreated PDB and can be used to track the response to treatment. If the patient has an elevated ALP due to biliary disease or liver dysfunction, a more specific marker of bone turnover can be used, such as bone-specific ALP, procollagen type 1 amino-terminal propeptide (P1NP) or urine N-terminal telopeptide (uNTx). Significantly higher levels of ALP and bone turnover markers are seen in patients with polyostotic PDB, familial cases and patients with skull involvement.14,16
A recent meta-analysis of 17 observational studies showed that P1NP has the highest correlation with disease activity before and after treatment.18 Total ALP and uNTx are recommended for following disease activity after treatment if P1NP is unavailable (Table 2).16
| Table 2. Bone turnover markers used in the assessment of Paget’s disease18 |
| Turnover marker |
Sensitivity for detecting PDB |
Use in monitoring treatment response |
| Bone formation marker |
| Total ALP |
69–100% |
Yes, unless liver dysfunction |
| Bone ALP |
82–100% |
Suggested if there is liver dysfunction |
| Procollagen type 1 amino-terminal propeptide |
77–100% |
Most sensitive test of treatment effect |
| Bone resorption marker |
| Urine N-terminal telopeptide of type I collagen |
94–100% |
Most sensitive test of treatment effect |
| ALP, alkaline phosphatase; PDB, Paget’s disease of bone |
An assessment of 25-hydroxyvitamin D is recommended to exclude vitamin D deficiency and secondary hyperparathyroidism as a cause for raised serum ALP. Vitamin D deficiency must be corrected before bisphosphonate therapy is given so that treatment-related hypocalcaemia is minimised.20
Radiological imaging
The diagnosis of PDB is confirmed by typical radiological findings (Table 3).
| Table 3. Radiographic changes appearing in each phase of the pagetic lesion16 |
| Phase of Paget activity |
Radiographic findings |
| Osteolytic |
- Osteoporosis circumscripta in skull
- Blade of grass or candle flame signs in long bones
|
| Mixed |
- Coarsened trabeculae and bony enlargement mixed with osteolytic zones
- Cotton wool appearance of the skull
- Diploic space widening (inner and outer calvaria tables)
- Vertebral frame sign
- Squaring of vertebrae
- Coarse vertebral trabecular thickening
- Ivory vertebrae
- Enlargement of the pubic rami and ischium
|
| Sclerotic |
- Frontal bone enlargement
- Cortical thickening and sclerosis of iliopectineal and ischiopubic lines
- Acetabular protrusion
- Lateral curvature of the femur
- Looser zones
- Banana and chalk transverse fracture in long bones
|
Plain radiography is recommended for diagnosis of PDB as the pagetic changes are easily recognisable. The characteristic features on X-ray are listed in Table 3 and illustrated in Figure 2. Typical findings of PDB are focal osteolytic lesions or advancing lytic wedges in long bones in the early stages, sclerotic changes associated with thickened trabeculae and cortices, bone expansion and deformity. Plain films can also be used to identify fractures and exclude metastatic disease as a differential diagnosis for lytic bone lesions.16
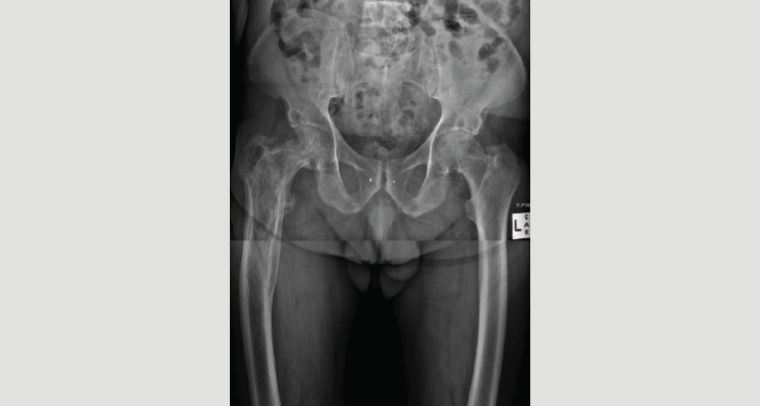
Figure 2. Plain X-ray of active Paget’s disease of bone in the right proximal femur of a man aged 75 years. Note the abnormal appearance of the femur, with expanded cortices and bowing deformity. One month after this X-ray was taken, the patient sustained a subcapital fracture, necessitating a hip replacement.
Bone scintigraphy (Tc-99m bone scan) is more sensitive than plain radiology for identifying areas of increased osteoblastic activity and can be used to assess the distribution of asymptomatic disease. This is important to identify bones that are at risk for local complications such as the long bones, base of skull and vertebrae.20
Management
The current management guidelines for PDB recommend that treatment should be reserved for symptomatic patients, while those who are asymptomatic be observed, as outlined in Figure 3.
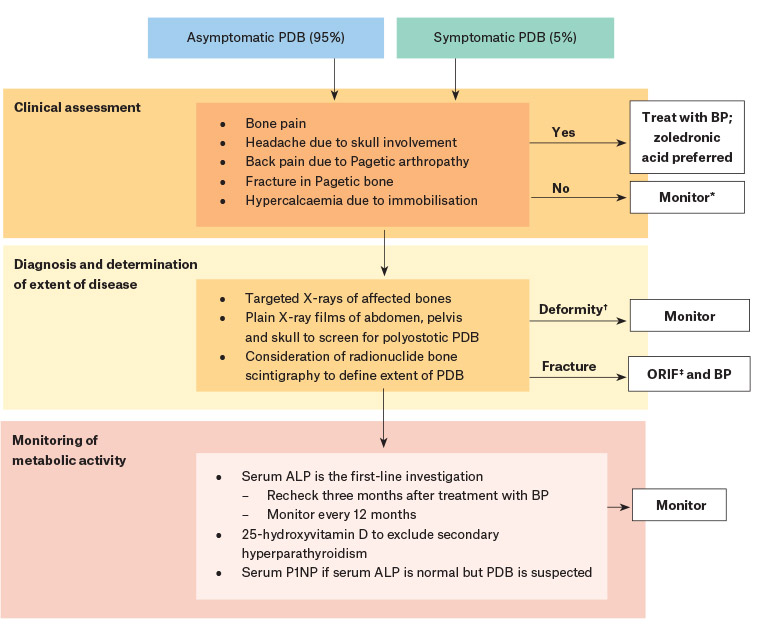
Figure 3. Management and treatment algorithm for Paget’s disease of bone16,20
*Monitor serum ALP every 12 months, while treatment is indicated for symptomatic PDB.
†There is insufficient evidence to recommend bisphosphonates to prevent bone deformity or progression of osteoarthritis in PDB.
‡ORIF is recommended in treating fractures of pagetic bone. There is insufficient evidence to support use of pre-operative bisphosphonates to reduce intraoperative blood loss.
ALP, alkaline phosphatase; BP, bisphosphonates; ORIF, open reduction and internal fixation; PDB, Paget’s disease of bone; P1NP, procollagen type 1 amino‑terminal propeptide
The treatment of choice for PDB is bisphosphonates, which are highly effective at suppressing the accelerated bone turnover by directly inhibiting osteoclasts (Table 4). They are the only agents that have been evaluated in randomised clinical trials to significantly reduce bone pain, accelerate the healing of lytic lesions, reduce bone turnover markers and improve quality of life when compared with placebo.16,21
| Table 4. Recommended bisphosphonates with dosing regimens20 |
| Medication |
Dosage |
| Zoledronic acid |
5 mg given as a single infusion over 15 minutes. Retreatment is seldom required within five years. |
| Alendronate |
40 mg/day for six months. Retreatment may be required between two and six years later. |
| Risedronate |
30 mg/day for two months. Retreatment may be required between one and five years later. |
While all bisphosphonates have been shown to be effective in PDB, zoledronic acid is most effective.
A randomised open-label trial comparing zoledronic acid with pamidronate that recruited 89 participants showed that a single intravenous infusion of 4 mg zoledronic acid was more likely to give pain relief than 30 mg pamidronate when given on two consecutive days every three months.22
Comparing zoledronic acid with risedronate, a recent randomised double-blind study involving 357 patients showed that a single dose of intravenous zoledronic acid was more likely to provide pain relief than risedronate sodium 30 mg daily orally for two months (relative risk = 1.36, 95% confidence interval [CI]: 1.06, 1.75; number needed to treat [NNT] = 7, 95% CI: 4, 24).23 In addition, clinical relapse – defined as a recurrence of bone pain – was less likely in the zoledronic acid group than the risedronate group (9.2%, compared with 25.2%). Furthermore, the rate of biochemical relapse – defined as a rise in bone turnover markers – was much lower in the zoledronic acid group (0.7%) when compared with the risedronate group (20%).24 This finding confirms that biochemical and clinical relapse in PDB are distinct entities,16 and both are suppressed more effectively by zoledronic acid than other bisphosphonates.
In a Cochrane review, bisphosphonates resulted in a 50.1% greater reduction in total ALP than placebo and were far more likely to normalise the total ALP (risk ratio [RR] = 9.96; 95% CI: 3.74, 26.58).21 A single dose of intravenous zoledronic acid was far more effective in normalising bone turnover than risedronate (347 participants: RR = 1.53, 95% CI: 1.33, 1.76; NNT = 3, 95% CI: 3, 5) or pamidronate (90 participants, RR = 2.57, 95% CI: 1.79, 3.70; NNT = 2, 95% CI: 1, 3).21
The duration of the effect on bone turnover is also longer after zoledronic acid than other bisphosphonates. In a long-term extension of the Paget’s Disease: Randomised Trial of Intensive versus Symptomatic Management (PRISM) study, 88% of patients treated with a single dose of 5 mg zoledronic acid intravenously still had a normal serum total ALP after five years’ follow-up, compared with 47% of patients treated with oral risedronate sodium.25,26
For the majority of patients with PDB who are asymptomatic, the question is whether treatment should be given to reduce bone turnover markers and prevent complications such as osteoarthritis and fractures. The PRISM study sought to answer this by comparing outcomes for asymptomatic patients given zoledronic acid to normalise bone turnover markers with symptomatic patients whose treatment aimed to reduce bone pain. This randomised study involved 502 participants over a seven-year period of follow-up.26 It showed that zoledronic acid had similar effects on quality of life and bone pain in both groups, but asymptomatic patients were more likely to experience fractures and orthopaedic procedures than symptomatic patients.26 This has led to the recommendation that bisphosphonate therapy should be reserved for use in symptomatic PDB and focus on symptom management rather than suppression of bone turnover.16,20
Denosumab is an alternative antiresorptive therapy used for patients with osteoporosis; however, it has been less studied in PDB than bisphosphonates. While case reports of its use in PDB show its effectiveness in reducing bone turnover markers for up to five months after administration, its effect on bone pain, fracture risk and progression of pagetic lesions is less clear.20 At this stage, it is not recommended as treatment for PDB.
Treatment response is best assessed by measuring serum total ALP 3–6 months after treatment and then annually once levels are normalised. If there are osteolytic lesions, the plain film should be repeated at 12 months to assess for improvement. A single infusion of zoledronic acid has long-term benefits, with sustained remission rates of 87% at 6.5 years.26 If symptoms recur and serum ALP rises above the normal range, retreatment with zoledronic acid should be considered.20,26
Conclusion
Paget’s disease is a common metabolic bone disorder that affects older patients. Zoledronic acid is highly effective in treating symptomatic disease, with high rates of long-term remission.