Hypothyroidism and hyperthyroidism are commonly encountered problems in clinical practice. General practitioners are well placed to be the primary clinicians overseeing the long-term management of patients with thyroid function abnormalities.
What are the causes of hypothyroidism?
The diagnosis of hypothyroidism relies on confirmation by laboratory testing. Primary hypothyroidism, caused by failure of the thyroid gland, is characterised by a decreased serum free thyroxine (FT4) level with an appropriately elevated serum thyroid stimulating hormone (TSH) level. Secondary hypothyroidism is a rare condition caused by hypothalamic or pituitary disease and characterised by a low serum FT4 level without an increased TSH level, which is low or even normal.
Causes of hypothyroidism are listed in Box 1. Globally, iodine deficiency remains the most common cause of hypothyroidism.1 Hashimoto’s thyroiditis (autoimmune thyroiditis) is the most common cause of primary hypothyroidism in Australia and most iodine-sufficient areas of the world.2 Hashimoto’s thyroiditis is characterised by gradual thyroid failure, with or without goitre formation, due to autoimmune-mediated destruction of the thyroid gland. The exact prevalence of primary hypothyroidism in Australia is unknown, but it is probably similar to that found in the USA, where hypothyroidism has been documented in 4.6% of the population, with 0.3% being clinical and 4.3% being subclinical.3 Approximately 10–20% of the Australian population have evidence of thyroid autoimmunity based on the presence of circulating thyroid autoantibodies,4 but prevalence may vary with age, sex and ethnicity.
| Box 1. Causes of hypothyroidism19 |
- Autoimmune lymphocytic thyroiditis (Hashimoto’s thyroiditis)
- Post-ablative therapy
- Radioiodine therapy
- Thyroidectomy
- Transient
- Subacute thyroiditis
- Postpartum thyroiditis
- Subtotal thyroidectomy
- Medication induced
- Thionamide (carbimazole, propylthiouracil)
- Lithium
- Amiodarone
- Interferon
- Immune checkpoint inhibitors
- Medications that interfere with thyroxine absorption (eg iron, calcium, cholestyramine, sulcralfate)
- Iodine associated
- Iodine deficiency
- Iodine induced (eg contrast load, Lugol’s iodine)
- Infiltrative
- Riedel’s thyroiditis
- Amyloid
- Haemochromatosis
- Scleroderma
- Neonatal/congenital
- Thyroid agenesis/ectopia
- Genetic disorders of thyroid stimulating hormone (TSH), TSH receptor, thyroid peroxidase, thyroglobulin, pendrin
- Transplacental passage of blocking TSH receptor antibody
- Secondary
- Hypothalamic or pituitary disease
- Other
- Thyroid hormone resistance
- Factitious (eg falsely elevated TSH due to heterophile antibodies)
|
Approach to management of subclinical hypothyroidism
Subclinical hypothyroidism, defined biochemically as an elevated TSH level accompanied by a normal FT4 level, is a very common finding in general practice. It is useful to measure thyroid peroxidase antibodies (anti-TPO) to identify underlying Hashimoto’s disease as the cause. People with Hashimoto’s thyroiditis have an increased risk of other autoimmune conditions including coeliac disease, vitamin B12 deficiency and Addison’s disease.
Most people with subclinical hypothyroidism will have minimal or no specific symptoms. It can be challenging to determine the extent to which mild thyroid dysfunction is causing a patient’s symptoms because of the high rate of some complaints (eg cold intolerance, weight gain, constipation, fatigue, hair loss and dry skin) in the general population.
As subclinical hypothyroidism progresses to overt hypothyroidism at a rate of approximately 5% per year, asymptomatic patients with subclinical hypothyroidism can usually be observed with annual thyroid function tests (TFTs).
Most guidelines recommend levothyroxine (LT4) treatment if TSH is >10 mIU/L.5 A lower TSH threshold is appropriate in younger individuals and during pregnancy. A TSH >2.5 mIU/L should prompt consideration of the need for LT4 therapy during pregnancy, and international guidelines recommend LT4 replacement when TSH is >4.0 mIU/L and the patient is anti-TPO positive.6 Based on the number of annual Pharmaceutical Benefits Scheme prescriptions for LT4 replacement therapy, it is likely that approximately one million people are being treated for hypothyroidism in Australia. It is probable that the vast majority of these patients are being treated for subclinical hypothyroidism.
A recent systematic review concluded that most non-pregnant individuals with subclinical hypothyroidism do not benefit from treatment.7 This review has further ignited debate regarding treatment thresholds and highlighted concerns about the potential for overtreatment.
Age-specific local reference ranges for TSH should be considered when establishing a diagnosis of subclinical hypothyroidism, particularly in older people. Unfortunately, these reference ranges are not generally available in Australia. A recent placebo-controlled, randomised, double-blind study failed to find any benefit from treatment of subclinical hypothyroidism (mean baseline TSH 6.4 mU/L) in 737 elderly patients.8 Most elderly patients with subclinical hypothyroidism should be carefully followed up with a ‘wait and see’ strategy, generally avoiding replacement therapy.
Figure 1 provides an algorithm to assist in decision making for LT4 replacement therapy for those with either overt or subclinical hypothyroidism.9
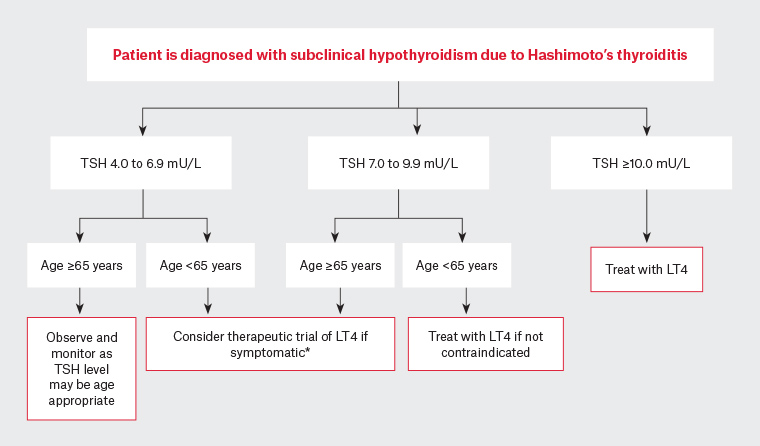
Figure 1. Algorithm for thyroid hormone replacement in adults with subclinical hypothyroidism due to Hashimoto’s thyroiditis9
*Patients who commence LT4 therapy for symptoms attributed to subclinical hypothyroidism should be reviewed after three or four months to assess response to treatment once the serum TSH returns to the reference range. If symptoms have not improved then LT4 therapy should generally be discontinued and the patient reviewed for other disorders.
LT4, levothyroxine; TSH, thyroid stimulating hormone
Reproduced with permission of Medicine Today from Hughes K, Eastman CJ, Hashimoto’s thyroiditis: How to spot the diagnosis and how to manage it, Med Today 2017;18(9):27–32.
Goals of hypothyroidism treatment
The goals of therapy for hypothyroidism include:
- amelioration of hypothyroid symptoms
- restoration of a euthyroid state
- avoidance of overtreatment.
Restoration of a euthyroid state can be readily accomplished in almost all patients by oral administration of LT4. The average full replacement dose of LT4 in adults is approximately 1.6 μg/kg body weight per day. Mildly elevated levels of FT4 may be seen if blood is taken in the first few hours after swallowing the medication. In general, it is best to base dosing decisions predominantly on TSH levels. Thyroxine has a long half-life of approximately seven days, and steady-state TSH concentrations are therefore not achieved for at least six weeks. Consequently, it is best not to repeat TFTs sooner than this after initiating LT4 therapy or changing the dose. The long half-life also enables different doses to be given on different days to provide the required total weekly dose. In elderly patients and those with known cardiac disorders, it is better to go ‘low and slow’, starting with 25–50 μg per day.
Because co-administration of food with the medication can impair LT4 absorption, the medication should be taken while fasting at least 30 minutes – and ideally 60 minutes – before breakfast. For patients unable to comply, bedtime dosing can be considered (three or more hours after the evening meal). Care should also be taken not to co-administer with supplements such as iron and calcium, which may reduce absorption. It is generally appropriate to target a TSH level within the reference range. The most common cause of failure to achieve normal TSH levels despite escalation of thyroxine doses is non-adherence with therapy. If a patient has ongoing symptoms suggestive of hypothyroidism and the serum TSH is confirmed by repeat measurement to be at the upper limits or above the reference range, it is reasonable to increase the dose and to aim for a serum TSH value in the lower half of the reference range. Some studies have suggested psychological wellbeing is better in patients with lower serum TSH concentrations.10
Combination thyroxine/triiodothyronine (T4/T3) therapy may have a limited role in the minority of patients who are dissatisfied with T4 monotherapy. Professional guidelines support the use of liothyronine (LT3) in combination with LT4 for those patients who have been properly screened and unambiguously have not benefited from LT4. If the serum TSH level stays within the reference range, replacing a small fraction of the LT4 dose by LT3 once or twice per day has not been associated with adverse drug reactions.11 Combination therapy should generally be initiated under specialist supervision with care to avoid overtreatment. The European Thyroid Association has published guidelines with the intent of enhancing the safety of combination therapy.12 Desiccated thyroid extract has been extensively used in the past and is widely promoted on social media sites, but it is not recommended by any of the current specialist society guidelines. Typically it has a T4-to-T3 ratio of 4:1 – providing much more T3 than the physiological ratio of approximately 13:1 to 16:1. The result is that thyroid extract will often produce supraphysiological T3 levels that may be associated with harm; it is contraindicated in pregnant patients or the elderly with cardiac disorders.
Potential adverse effects of excessive thyroid hormone replacement
Because many symptoms of hypothyroidism are nonspecific, patients often think that their LT4 dose is inadequate, such as when they feel excessively tired or gain weight. This can lead to an individual requesting an LT4 dose escalation or self-escalating their dose, causing a suppressed serum TSH level.
TSH levels of <0.4 mIU/L have been associated with osteoporosis and atrial fibrillation in people aged >60 years of age.13 In one study, patients aged >65 years with serum TSH levels <0.1 mIU/L, the majority of whom were taking LT4, had a threefold increase in the risk of atrial fibrillation over a 10-year observation period when compared with euthyroid controls.14
The risk for low bone density and fractures is also elevated in postmenopausal women taking excessive LT4. In a cohort of women aged >65 years, women with a low TSH level (≤0.1 mU/L) had a threefold increased risk for hip fracture (relative hazard: 3.6; 95% confidence interval [CI]: 1.0, 12.9]) and a fourfold increased risk for vertebral fracture (odds ratio: 4.5; 94% CI: 1.3, 15.6) when compared with women who had normal TSH levels (0.5–5.5 mU/L).15 In another prospective cohort study of >230,000 women, there was an increased incidence of major osteoporotic fracture (hip, spine, humerus, forearm) in patients with low TSH levels (<0.3 mU/L; 13.5%, compared with 6.9% in those with normal TSH levels).16
Potential adverse effects of inadequate thyroid hormone replacement
The adverse effects of thyroid hormone deficiency are often nonspecific and may include cognitive impairment, hyperlipidaemia and progression of cardiovascular disease. Patients with overt hypothyroidism should generally have the LT4 dose adjusted to achieve a normal TSH level to avoid these potential adverse effects. In extreme situations, myxoedema coma could eventuate.
Management of hypothyroidism during pregnancy
Thyroxine production increases early in gestation by 25–50% in response to human chorionic gonadotrophin (hCG) stimulation of the normal thyroid gland and increased oestrogen-stimulated synthesis of thyroid hormone–binding proteins. Accordingly, patients with hypothyroidism who are maintained on LT4 therapy should increase the dosage of their medication initially by approximately 25% as soon as pregnancy has been confirmed. This increase can often be easily achieved with an extra two daily doses each week. Regular monitoring of serum TSH and FT4 levels is recommended, particularly during the first half of gestation, to adjust the LT4 dosage to maintain these parameters within the normal pregnancy reference range (0.1–2.5 mIU/L). A therapeutic and monitoring regimen should apply to women diagnosed with either overt or subclinical hypothyroidism during pregnancy. Soon after delivery, the LT4 dosage must be reduced to the original prepartum replacement dosage.
What are the causes of hyperthyroidism?
Common causes of hyperthyroidism are listed in Table 1. Investigations to determine the cause of thyrotoxicosis should routinely include TSH, FT4, FT3 and thyroid antibodies including thyroid receptor antibodies (TRAb). C-reactive protein should be checked if subacute thyroiditis is suspected (indicated by a painful, tender thyroid). Thyroid uptake scans are useful if the diagnosis is not clear based on clinical features and bloods tests. Thyroid sonography has a limited role in evaluation of patients with thyrotoxicosis and is not necessary as part of routine assessment.
| Table 1. Common causes of thyrotoxicosis and main diagnostic features |
| Cause |
Aetiology |
Uptake scan finding |
Laboratory thyroid autoantibody results |
| Graves’ disease |
TRAb stimulate thyroid hormone production and development of a
diffuse goitre |
Normal or diffusely increased isotope uptake |
Presence of TRAb is diagnostic |
| Toxic multinodular goitre or toxic adenoma |
Autonomous nodules produce thyroid hormone without TSH stimulation; hyperthyroidism may be precipitated or exacerbated by exposure to excess amounts of iodine |
Increased isotope uptake into toxic nodules with reduced uptake into surrounding normal thyroid tissue |
Typically, a gradual progression towards the hyperthyroid state over several years and thyroid autoantibody negative |
Painless thyroiditis or
postpartum thyroiditis |
Release of preformed thyroid hormone due to autoimmune destruction of
thyroid tissue |
Reduced or absent isotope uptake |
Anti-TPO antibodies and/or antithyroglobulin antibodies are present |
| Painful, subacute thyroiditis |
Release of preformed thyroid hormone due to virally mediated destruction of thyroid tissue |
Reduced or absent isotope uptake |
Anti-TPO and antithyroglobulin antibodies usually not detected and CRP/ESR elevated |
| Amiodarone-induced thyrotoxicosis |
Type 1 – iodine induced in people with underlying autonomous nodules or Graves’ disease; Type 2 – destructive thyroiditis |
Usually reduced or absent isotope uptake, but often not helpful |
Nil characteristic laboratory results; recommend checking anti-TPO antibodies and TRAb to look for competing causes |
| Exogenous thyroid hormone excess: Iatrogenic, intentional or factitious |
Excess ingestion of thyroid hormone |
Reduced or absent isotope uptake |
Nil characteristic laboratory results |
| Anti-TPO, thyroid peroxidase antibodies; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; TRAb, thyroid receptor antibodies; TSH, thyroid stimulating hormone |
Management of Graves’ disease
Graves’ disease has three different treatment modalities:
- antithyroid medications (thionamides) that block the synthesis of thyroid hormones
- radioactive iodine ablation (I-131)
- thyroidectomy.
Each of these modalities of therapy is a satisfactory treatment but none is ideal. The advantages and disadvantages of different types of treatment are listed in Table 2. Individual patient factors will influence the choice of therapy, and management decisions should involve discussion of the values and preferences of the patient. Administration of beta-blocker medication (eg propranolol, atenolol, metoprolol) is recommended as initial, short-term symptomatic therapy in all patients with moderate-to-severe symptomatic thyrotoxicosis.
| Table 2. Advantages and disadvantages of Graves’ disease treatment options |
| Treatment |
Advantages |
Disadvantages |
| Thionamides |
- Allows possibility of a durable remission and preservation of endogenous thyroid function
- Low cost
|
- Side effects – rash, pruritus, gastrointestinal, agranulocytosis and hepatitis, usually occurring early in the course of therapy
- Risk of birth defects if pregnant
- Frequent monitoring required
|
| Radioiodine |
- Permanent resolution of hyperthyroidism
- Low cost
|
- Permanent hypothyroidism in most cases
- Radiation exposure to salivary glands
- Potential adverse effects on fertility
- Risk of exacerbation of Graves’ orbitopathy
|
| Thyroidectomy |
- Rapid, permanent resolution of hyperthyroidism
- May help improve Graves’ orbitopathy
- Provides relief of compressive symptoms if large goitre is present
|
- Permanent hypothyroidism
- Risks of surgery and anaesthesia
- Risk of hypoparathyroidism and recurrent laryngeal nerve injury
- High cost in comparison to other treatments
|
Antithyroid medications are the usual first-line treatment for patients with Graves’ disease and are generally favoured because they allow the possibility of achieving a durable remission without the need for lifelong thyroid hormone replacement. Patients with mild hyperthyroidism, a minimally enlarged thyroid and/or only modestly elevated TRAb levels are particularly good candidates for a trial of thionamide therapy as they have the best chance of achieving a durable remission. Most individuals will need treatment for 12–18 months, with treatment continuing until the TRAb becomes undetectable. Carbimazole is the favoured thionamide as it has less hepatotoxicity than propylthiouracil (PTU). However, PTU is preferred during the first trimester of pregnancy and in treatment of thyroid storm because it inhibits conversion of T4 to T3. PTU may also be used for people with adverse reactions to carbimazole. Agranulocytosis is a rare but serious adverse effect of thionamide medications. Before commencing thionamides, all patients should have baseline full blood examination and liver function tests performed. Any patient taking thionamides who develops fever, sore throat or other signs of sepsis should have an urgent assessment of white cell count and liver function. It is worth discussing the risk of agranulocytosis when prescribing thionamides so patients are aware to attend for a blood test if infective symptoms develop.
Long-term thionamide medication has generally been discouraged because of challenges with compliance and potential side effects; however, some patients and clinicians express a preference for thionamide, wanting to avoid permanent hypothyroidism from either radioactive iodine ablation or thyroidectomy.17 A course of antithyroid medication is recommended to achieve euthyroidism before the patient is treated definitively with either radioiodine or thyroidectomy.
Definitive treatment with radioiodine is generally considered in the following settings:
- failure to achieve a durable remission despite prolonged or recurrent courses of thionamide therapy
- recurrent Graves’ disease
- individuals who are unable to tolerate thionamides because of adverse effects.
The cure rate (achieving euthyroid or hypothyroid state) following the oral administration of a 555 Mbq dose of radioactive iodine is approximately 75% at 12 months. Radioiodine typically takes 3–6 months to induce a hypothyroid state, and some individuals will need more than one dose. There is lingering concern about possible adverse effects of I-131 ablation on fertility in women and a potential small increased risk of malignancy associated with radiation exposure. Radioiodine carries the risk of exacerbating Graves’ orbitopathy and so is best avoided in patients with significant thyroid eye disease. Corticosteroids should be given prophylactically at the time of radioiodine therapy to reduce the risk of a flare of orbitopathy in individuals with mild orbitopathy.
Thyroidectomy is generally the preferred option in the following settings:
- moderate-to-severe Graves’ orbitopathy
- women who desire a pregnancy within the next 6–12 months
- large goitres causing compressive symptoms or goitres with significant retrosternal extension
- where thyroid malignancy is suspected
- when patients are fearful of taking radioactive medication.
Thyroidectomy should only be performed by a high-volume surgeon as complication rates are lower in the hands of an experienced surgeon.
None of the available therapeutic options for the management of Graves’ disease has been able to re-establish normal thyroid function in all patients. Regardless of the treatment chosen, up to 25% of patients report not feeling fully recovered after their treatment, mostly because of persistent fatigue and eye symptoms.18 Weight loss is a common feature of hyperthyroidism, and many patients gain considerable weight after treatment of their hyperthyroidism. Patients should be counselled regarding the risk of weight gain; appropriate early dietary modification may help to minimise weight gain.
Management of toxic nodules
Toxic nodules are a common cause of mild hyperthyroidism, generally progressing slowly over many years. In the early stages, a subnormal TSH level is frequently the only biochemical abnormality. Risks of osteoporotic fractures and atrial fibrillation increase, particularly in the elderly, as the TSH levels falls below 0.1 mIU/L; therefore, this is a common threshold for considering treatment (even when the FT4 and FT3 concentrations remain within the reference range). Exposure to large iodine loads, as occurs with iodinated contrast, may precipitate a transient increase in severity of hyperthyroidism. Diagnosis is usually confirmed by the appearance on a radionuclide scan (Figure 2).
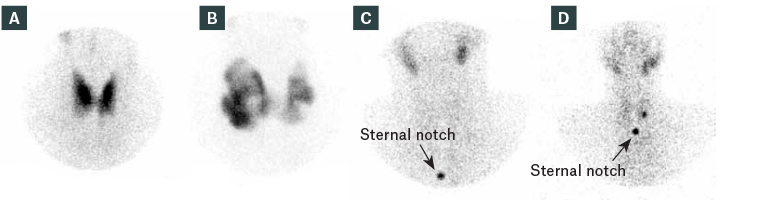
Figure 2. Uptake scan appearances of thyroid conditions20
A. Graves’ disease; B. Multinodular goitre; C. Thyroiditis; D. Autonomous nodule
Reproduced with permission of The Royal Australian College of General Practitioners from Lee JC, Harris AM, Khafagi FA, Thyroid scans, Aust Fam Physician 2012;41(8):584–86.
Radioiodine is generally the preferred treatment for toxic nodules as it provides the best chance of achieving the euthyroid state without the need for medication. Surgery is preferable in patients with very large goitres and/or significant compressive symptoms and for those who do not want to have radiation therapy. Long-term thionamide therapy is an option, particularly in elderly people or in individuals who prefer to avoid radiation or surgery. Percutaneous ethanol therapy or laser therapy are available in some parts of the world, but their availability in Australia is very limited currently.
Management of thyroiditis
Thyroiditis may have multiple different causes, including autoimmune (eg painless lymphocytic and postpartum thyroiditis), viral (eg subacute thyroiditis) and medication induced (eg amiodarone). Thyrotoxicosis associated with thyroiditis is typically self-limited. Antithyroid (thionamide) medication therapy is generally ineffective and inappropriate. Beta-blockers are indicated for symptomatic relief in patients with significant palpitations, tachycardia and tremor. Corticosteroids may have a role in people with persistent, painful subacute thyroiditis and in people with type 2 destructive thyroiditis associated with amiodarone therapy.
Hyperthyroidism associated with thyroiditis is attributable to the release of preformed thyroid hormone from the inflamed thyroid. The thyrotoxic phase may be followed by a hypothyroid phase, so monitoring of thyroid function tests is prudent. The hypothyroid phase is usually transient; therefore, thyroxine replacement may not be required. If LT4 is considered necessary, it can usually be weaned within 3–6 months. The rate of permanent hypothyroidism associated with thyroiditis will depend on the underlying cause. Females with a history of thyroiditis should be encouraged to have TSH checked prior to attempting conception and during the first trimester of pregnancy as they are at risk of developing hypothyroidism during pregnancy.
Conclusion
Thyroid function disorders are commonly encountered in general practice. Long-term management requires an understanding of the range of causes and benefits from a shared decision-making approach, with discussion about potential risks and benefits of therapy.
Key points
- Thyroid function abnormalities are commonly encountered in general practice, occurring much more often in females than males, with close to a million people being treated for hypothyroidism.
- Treatment of established hypothyroidism should be with LT4, in preference to other forms of thyroid hormone replacement, with goals of ameliorating symptoms, achieving the euthyroid state and avoiding overtreatment.
- Decisions about who and when to treat for hypothyroidism can be challenging and may benefit from a shared decision-making approach with discussion about potential risks and benefits of therapy.
- Hyperthyroidism, even when subclinical, carries long-term risks of osteoporosis and atrial fibrillation, particularly in the elderly, and generally should not be left untreated.
- Choice of treatment modality for hyperthyroidism should be patient centred and dependent on underlying pathology, patient preference and availability of expert surgical care.