Haematuria is one of the most common urological presenting complaints – either incidentally or symptomatically detected – and is important as it can be a strong indicator of underlying malignancy.1,2 Previous studies suggest the positive predictive value of haematuria being secondary to an underlying urological malignancy is as high as 11%, highlighting the importance of appropriate primary care work-up and prompt specialist referral where indicated.3 Bleeding can originate from anywhere along the urinary tract, from kidneys to the external urethral meatus, and can be broadly divided into five categories (Table 1). It may be classified on the basis of degree of haematuria; gross haematuria refers to blood that is visibly seen in the urine at a macroscopic level, in comparison to non-visible, or microscopic, haematuria. It is further characterised by presence or absence of symptoms. Microscopic haematuria is most commonly defined as >3 red blood cells per high-power field on urinary microscopy.4,5 Urine dipstick analysis is not sufficient to diagnose microscopic haematuria; however, it should prompt formal analysis with urinary microscopy.
| Table 1. Aetiology of haematuria |
| Aetiology |
Cause |
Origin |
| Transient |
Exercise induced |
Bladder |
| Trauma |
Urethra |
Sexual intercourse
Pelvic organ prolapse
Vaginal atrophy |
Vagina/urethra/uterus |
| Malignant |
Urothelial cancer |
Bladder/ureter/renal pelvis |
| Prostate cancer |
Prostate |
| Renal cell carcinoma |
Kidney |
| Infectious/inflammatory |
Pyelonephritis |
Kidney |
Lower urinary tract infection
Radiation cystitis
Foreign body |
Bladder |
| Urethral caruncle |
Urethra |
| Renal medical disease |
Immunoglobulin A nephropathy
Thin basement membrane disease
Hereditary nephritis |
Kidney |
| Obstructive |
Urolithiasis |
Kidney/ureter |
| Benign prostatic hyperplasia/prostatic regrowth |
Prostate |
All patients presenting with gross haematuria require comprehensive evaluation to rule out malignant causes. This includes imaging of the urinary tract and referral to a urologist for cystoscopic examination of the bladder.2 Microscopic haematuria is a common incidental finding that can indicate underlying significant pathology; however, ultimately only 30% of patients are found to have an identifiable cause, either malignant or benign.4,5 Initial history and examination are important in patients with microscopic haematuria in order to prevent over-investigation by using risk stratification.
History and physical examination
Initial evaluation of a patient presenting with haematuria should incorporate careful history-taking and physical examination. History of risk factors for urological malignancy should be considered, with male sex, smoking history and increased age the greatest risk factors (Box 1).6 Occupational exposures – including dyes, benzenes, and aromatic amines – as well as prior history of cyclophosphamide exposure or radiotherapy should be carefully sought out in history-taking and flagged in referral pathways. It is important to be aware that commencement or use of anticoagulation and antiplatelet agents does not exclude a patient from requiring further evaluation as, although this may worsen the degree of haematuria, an underlying cause may have merely been unmasked. Irritative lower urinary tract symptoms (LUTS) such as urinary frequency, dysuria and urgency may reflect underlying urinary tract infection or an inflammatory process. It is important to acknowledge that a small but significant number of patients with underlying malignancy will present solely with LUTS, particularly patients with bladder carcinoma in situ (CIS).2 Patients with obstructive pathology often report symptoms of hesitancy, incomplete emptying and reduced urinary flow, and in men may be indicative of the most common cause of bladder outlet obstruction: benign prostatic hypertrophy.7
| Box 1. Risk factors for urinary tract malignancy in patients with haematuria 5,6,20–22 |
- Age
- History of gross haematuria
- Irritative lower urinary tract symptoms
- Smoking (current or past history)
- Occupational exposure (dyes, benzenes, aromatic amines)
- Cyclophosphamide exposure
- History of chronic urinary tract infection
- History of pelvic irradiation
|
Abdominal and urogenital examination as well as blood pressure assessment is indicated, as guided by history, in patients presenting with haematuria. Elevated blood pressure may be present in patients with glomerulopathic causes of haematuria. Renal angle or flank tenderness may indicate renal colic. The external genitalia should be inspected to exclude pathology that patients may not be forthright in offering because of sensitivity of the topic. Speculum examination in female patients may be included to exclude vaginal, cervical and uterine sources of bleeding. Digital rectal examination and prostate specific antigen testing should be considered in men over the age of 50 years to evaluate for prostate cancer, which can present with haematuria in advanced stages.
Initial investigations
Prior to referral to urology services, initial investigations to identify benign and transient causes for haematuria (particularly microscopic) are required. Patients suspected of having contaminated urine specimens (eg due to menstruation, trauma or urogenital atrophy in female patients) should have repeat urine microscopy performed once the suspected underlying condition is resolved or treated. Urine microscopy and culture should be reviewed for presence of squamous cells, leukocytes or organism culture. It is recommended that urinary tract infection be treated and repeat urine microscopy performed 6–12 weeks post-treatment to ensure resolution of haematuria, or alternatively onward referral for persistent haematuria. Caution should be exercised regarding patients presenting with haematuria and irritative urinary tract symptoms without proven infection as this has been associated with underlying malignancy such as bladder CIS.2 Biochemical investigations including renal function, coagulation and haemoglobin should be performed to rule out any gross biochemical instability.
Upper tract imaging is indicated prior to referral to urology services where possible to assist in guiding treatment. Either computerised tomography with intravenous pyelogram (CT IVP) or renal tract ultrasonography are the imaging modalities of choice, each with benefits and limitations. CT IVP is the gold standard for assessment of the urinary tract for malignancy; however, it exposes the patient to risk of contrast allergy and radiation, as well as increased costs.8,9 In younger patients (ie those aged <50 years), or those with significant renal impairment, renal tract ultrasonography is typically sufficient, providing adequate evaluation of the kidneys in a less costly and minimally invasive approach without the added radiation exposure or risk of contrast-induced nephropathy. Ultrasonography is, however, user dependent and does not provide detailed views of the ureters and bladder and therefore is less sensitive in detection of urolithiasis or urothelial lesions.9 CT IVP should be seen as the imaging modality of choice for older patients, those with risk factors for malignancy, and those with persistent haematuria.
Urinary cytology is often included in the investigative work-up of patients with both macroscopic and microscopic haematuria; however, guideline recommendations regarding when it should be used are varied. Cytology has a high sensitivity in the detection of high-grade bladder tumours, particularly CIS; however, it has low sensitivity for low-grade tumours.2 Overall, it is not recommended as a sole diagnostic tool but can be a helpful adjunct for patients who are undergoing cystoscopy, in particular those with risk factors for upper tract cancer or CIS.10 To perform cytology, patients are provided with three urine specimen jars and are asked to provide three mid-morning midstream urine samples on three consecutive days.
Urology and nephrology referral
Urological referral is recommended for all patients with gross haematuria, while patients presenting with microscopic haematuria should undergo a risk-stratified approach (Figure 1). Updated guidelines by the American Urological Association in 2020 recommend that low-risk patients with microscopic haematuria undergo shared decision making, with a repeat urinalysis within six months thought to be appropriate.
5 Low risk is defined as age <50 years, never or <10 pack-year history smoker, <10 red blood cells per high-power field, and no other risk factors. Recent studies have suggested female patients are more likely to experience a delay in referral and diagnosis of urological malignancy.
11,12 Although the exact reason for this is unclear, it is thought that initial management of presumed infection, menstruation, repeat testing and hesitance to over-investigate are contributing factors.
13
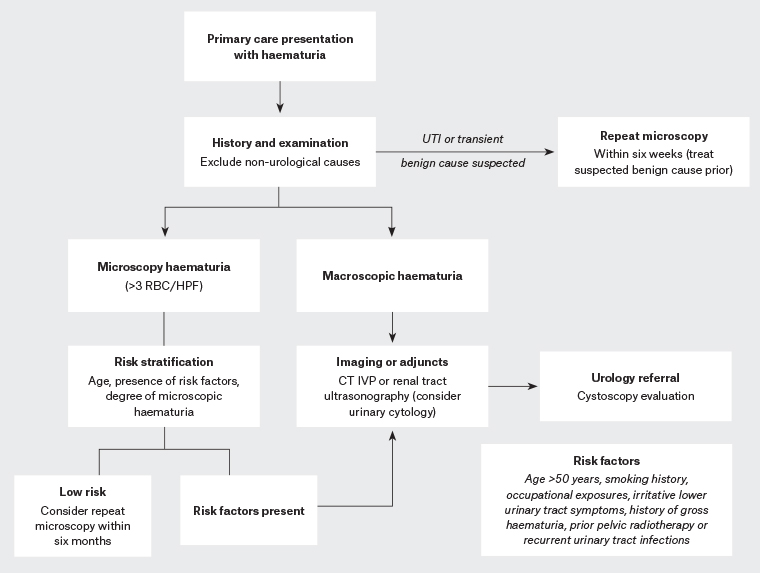
Figure 1. Algorithm for initial investigations and management of haematuria
CT IVP, computerised tomography with intravenous pyelogram; HPF, high-power field; RBC, red blood cell; UTI, urinary tract infection
Nephrology referral may be indicated for patients with evidence of glomerulopathic causes of haematuria. This includes proteinuria, dysmorphic red blood cells or casts on urinary microscopy, as well as renal insufficiency or hypertension in those aged <40 years.5 Isolated haematuria in otherwise well patients under the age of 40 years is often due to underlying mild glomerulonephritis with low risk of progression.14 Despite glomerulopathic causes of haematuria on urinary microscopy, exclusion of urological causes of haematuria with upper tract imaging and cystoscopy is still essential; therefore, initial referral to urological services from primary care is still appropriate in conjunction with nephrology referral.15
New directions for haematuria assessment
Direct access cystoscopy pathways
Concern for undue delay in diagnosis of significant urological malignancy heralded by haematuria has prompted the development of direct access cystoscopy pathways in several healthcare organisations.16 This pathway allows the patient to be streamlined from referral directly into cystoscopy booking, a referral pathway that has been in place for bowel cancer screening and colonoscopy for many years.17 Pitfalls of this referral pathway are in those patients who have transient and benign causes for their haematuria that would benefit from repeat testing prior to urological referral. Cystoscopy remains the gold standard for evaluation of the bladder; however, it is an invasive test and therefore should not be performed without sensible indication.
Genetic urinary biomarkers
Novel genetic urinary biomarkers are an emerging technology whereby bladder cancer screening, surveillance of bladder cancer and assessment for haematuria may be performed in a non-invasive manner in the future.18 Currently, no available urinary biomarker test is acceptable in replacing cystoscopy; however, use as an adjunctive test to monitor for missed tumours, in particular CIS, is emerging.19
Follow-up of patients with negative haematuria work-up
Patients commonly will be referred back to their general practitioners for ongoing care following negative urological haematuria work-up. In many instances, no further episodes of haematuria may occur. However, in the event of recurrent gross haematuria, timely referral back to urology services is prudent to re-evaluate for potentially missed lesions.
Repeat urinary microscopy is recommended at 6–12 months; if negative, no further evaluation is necessary following two negative analyses.5 Persistent microscopic haematuria at 12 months following a negative urological work-up should prompt shared decision making regarding the need for additional intervention.5 In patients yet to have a nephrological work-up, this should be considered, especially in the presence of renal impairment and/or proteinuria.14,15 Persistent microscopic haematuria without proteinuria or presence of other risk factors can be monitored annually with urinary microscopy, renal function and blood pressure assessment. Re-evaluation should be prompted if new symptoms occur; however, <1% of patients with initial unremarkable investigation for microscopic haematuria have been shown to go on to develop malignancy.4
Conclusion
Both microscopic and gross haematuria are very common presentations in primary care and an important heralding signal for potentially significant underlying pathology. Incidental detection of haematuria on routine assessment should prompt further evaluation, starting with history-taking and examination. Prior to referral to urological services, obtaining urinary tract imaging (either CT IVP or renal tract ultrasonography) and urinary cytology provides important adjunctive information to guide further evaluation. Presence of risk factors flagged in the referral pathway ensures timely assessment and therefore diagnosis of potential pathology. A significant proportion of patients with haematuria will have no identifiable cause found; therefore, having a framework for this demographic of patients is important in alleviating anxiety and avoiding unnecessary over-investigation while keeping in mind important triggers to prompt re-evaluation.
Key points
- Prior to urological referral for cystoscopy:
- positive dipstick haematuria should be confirmed with formal urinary microscopy
- imaging of the urinary tract should be obtained with either CT IVP or renal tract ultrasonography
- urinary cytology (three consecutive mid-morning midstream urine samples) can be considered, especially in patients with macroscopic haematuria or presence of risk factors for malignancy.
- Anticoagulant and antiplatelet agent commencement or use does not exclude a patient from requiring a comprehensive haematuria work-up.
- Urinary tract infection should be treated, and repeat urine microscopy performed after 6–12 weeks interval to ensure resolution of haematuria.
- Referral to nephrology may be considered for patients with microscopic haematuria and presence of renal impairment and/or proteinuria.
- Up to 70% of patients with haematuria may have no cause identified.