Since the initial report of COVID-19 in December 2019, understanding of the disease is evolving rapidly. The pathogenesis is better understood, and multiple vaccines are now available to contain the disease worldwide. This article focuses on the renal aspects of COVID-19.
From a primary care and renal perspective, three groups of patients that are likely to present to the general practitioner include:
- an individual with normal renal function who develops COVID-19
- an individual with impaired renal function (ie chronic kidney disease [CKD] stages 1–5) who develops COVID-19
- an individual who is on haemodialysis or peritoneal dialysis or has had renal transplantation who is exposed to SARS-CoV-2.
The acquisition of infection and the initial patterns of presentation are not different in the three groups, based on the current understanding of clinical presentation.
There is extensive discussion of the general aspects of COVID-19 elsewhere.1,2
Renal disease and COVID-19
COVID-19 can affect the kidney through:
- systemic processes that result in acute kidney injury (AKI) and multiorgan failure – seen with severe COVID-19
- direct cytopathic effect.
AKI is the most common form of renal involvement in COVID-19 and is associated with an increased risk of mortality and morbidity. Reported rates of AKI development vary across countries, with rates higher outside of China: 1–29% from China, 19–43% from the USA; within the UK, the nationwide rates were variable as well: 20% in April 2020, and 27% in July 2020.3 Almost all the data are related to hospitalised patients and therefore inherently reflect the more severe spectrum of COVID-19 disease.
From a primary care perspective, it leaves open the possibility that milder forms of AKI may not have been recognised.
A variety of indirect mechanisms lead to AKI in the setting of COVID-19.4 Hypovolaemia, either from diarrhoeal loss or insensible loss from hyperpyrexia, could lead to tubular injury. Development of secondary infections can cause sepsis-related AKI. Medications are another important cause of AKI – whether it be direct cytotoxicity, such as with contrast exposure, or through the development of interstitial nephritis. Rhabdomyolysis has been described in patients with COVID-19, which can, in turn, also lead to AKI. The complex communication that occurs via the release of cytokines and other signalling factors that are described with acute respiratory distress syndrome can also lead to AKI. Finally, the existence of premorbid conditions such as diabetes, chronic obstructive airway disease (COAD) and pre-existing CKD are likely to affect outcomes in patients with COVID-19.
Evidence of direct cytopathy from the virus as evidenced from histopathological data is currently limited. Severity of illness, the need for anticoagulation, and logistical difficulties related to the need to prevent viral transmission limit renal biopsies in patients with COVID-19-related AKI. An autopsy series from China on 26 patients with severe COVID-19 disease showed significant acute tubular injury.5 Direct identification of viral inclusion particles by electron microscopy within the kidney tissue is more variable, with positive and negative studies. Viral particles have been reported within the tubular epithelium as well as within podocytes on electron microscopy. Focal segmental glomerulosclerosis (FSGS) is a podocyte disease, and the collapsing glomerulopathy variant of the disease has been described in patients with COVID-19. It can be the consequence of direct viral infection among other factors; APOL1 alleles are a genetic risk factor for FSGS and are seen more commonly in patients with African ancestry.6
The presence of angiotensin converting enzyme 2 (ACE2) receptors on the endothelial cells,4 podocytes and proximal tubular cells suggest a role for direct viral tropism in the development of AKI.7 Polymorphisms in the membrane-bound ACE2 receptor, to which the spike protein of SARS-CoV-2 virus binds for cellular entry, could potentially affect the severity of the disease. Endothelial injury characterised by microvascular damage with endothelial dysfunction, coagulopathy and attendant complement activation leading to thrombotic microangiopathy are additional mechanisms of renal injury.7,8 There was interest recently as to whether use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers could alter the course of COVID-19. Evaluation of the evidence suggests that use of either agent does not affect the outcome of disease, and the current recommendation is that these agents should not be discontinued in patients with COVID-19.9
The ways in which COVID-19 can lead to AKI are outlined in Figure 1.
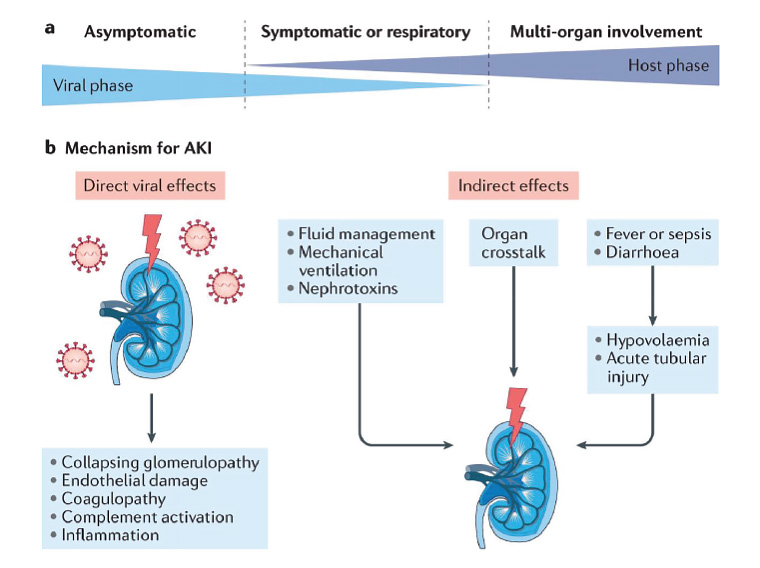
Figure 1. Acute kidney injury (AKI) in COVID-19 is multifactorial in its pathogenesis, arising from direct cytopathic effects as well as due to indirect effects from injury elsewhere in the body.
Reproduced with permission from Nadim MK, Forni LG, Mehta RL, et al, COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) workgroup, Nat Rev Nephrol 2020;16(12):747–64, doi: 10.1038/s41581-020-00356-5, licensed under CC BY 4.0.
Outcome of AKI in patients with COVID-19
From a set of New York hospitals, Chan et al reported 46% incidence of AKI in 3993 hospitalised patients with COVID-19 and 50% in-hospital mortality in those patients who developed AKI. Eighty-eight per cent of those with AKI who survived did not recover kidney function completely.10 Another US study that followed 182 patients who developed AKI as a result of COVID-19 reported that 82.4% recovered renal function comparable to those with AKI without COVID-19. However, in those patients who did not recover renal function, the rate of decline of estimated glomerular filtration rate was faster, suggesting the need for closer follow-up.11
Outcome of COVID-19 in patients with CKD from glomerulonephritides
The newly created International Registry of COVID Infection in Glomerulonephritis (IRoc-GN) identified 40 patients across North America and Europe who developed COVID-19. Mortality was higher in patients with CKD from pre-existing glomerulonephritis when compared with patients with COVID-19 without kidney disease (15%, compared with 5%); AKI rates were also higher in the group with pre-existing kidney disease than in the general population (39%, compared with 14%).12
The higher mortality and morbidity rates led the European Renal Association to devise a risk-stratified approach for patients with CKD during the current pandemic.13
Outcomes of COVID-19 in patients receiving dialysis
In a UK study, 36% of patients receiving dialysis with COVID-19 were managed as outpatients, initially dialysing in hospital isolation facilities and, following clinical improvement, in satellite units. Sixty-four per cent needed hospital admission. Patients who were not Caucasian and those with diabetes were more likely to be affected by SARS-CoV-2.10
In general, patients receiving haemodialysis deteriorated quickly, with the cumulative incidence of death rising steadily during the first 23 days of admission.14 Of the patients receiving haemodialysis who developed COVID-19, 22.8% died. Age was a significant factor; mortality was 11% in those under 50 years of age, compared with 32.2% in those over 80 years of age. Those who were breathless on admission had a hazard ratio of 2.32 (1.29, 4.17) for risk of death.
Fortunately, Australia has been spared a heavy onslaught from COVID-19 when compared with other nations as a result of vigilant public health units in the various states of the Commonwealth. As at 2 March 2021, there had been only 13 patients receiving dialysis who had been diagnosed with COVID-19 in Australia. However, the mortality rate has been high, as seven of these patients died.15
Outcomes of COVID-19 in renal transplant recipients
At this stage of the pandemic, in the absence of registry data, we are dependent on data from smaller studies. A French study involving two hospitals in Paris, who were following 1219 renal transplant recipients, reported a higher incidence of SARS-CoV-2 infection in these patients when compared with the general population (5%, compared with 0.3%). COVID-19 was associated with non-white ethnicity, obesity, asthma and chronic obstructive pulmonary disease, and diabetes. Mortality rate was high – 1% in the general population, compared with 24% in renal transplant recipients with COVID-19.16 In Australia, as at 2 March 2021, 21 renal transplant recipients developed COVID-19, and two of these patients died.15
Implications for primary care practitioners
Based on available evidence, most AKI associated with COVID-19 appears to develop in patients who have a severe form of COVID-19. There is not enough information on the renal outcomes in individuals with mild-to-moderate disease. The ability of SARS-CoV-2 virus to infect endothelial cells, podocytes and proximal tubular epithelial cells would lead one to expect problems with hypertension and proteinuria in the longer term. In survivors of severe COVID-19 with AKI, renal recovery is often incomplete, and managing CKD is an expected outcome for primary care.
When an individual has symptoms suggestive of SARS-CoV-2 infection, the current public health screening recommendations for SARS-CoV-2 infection will suffice as a first step, irrespective of the patient’s renal function. It is important to identify individuals who are at high risk of developing severe COVID-19 – those with risk factors such as old age, presence of cardiovascular disease, diabetes, COAD, kidney disease, immunosuppression and obesity, as well as relevant ‘high-risk’ socioeconomic factors.17 In these individuals, optimising fluid management, monitoring serum creatinine and urine output, avoiding and addressing hypoglycaemia, avoiding radiocontrast media for imaging wherever possible and avoiding other nephrotoxins would be part of the management strategy. It is important to be aware of the hypercoagulable state induced by COVID-19.
There are no specific measures to prevent COVID-19-related renal disease. Recommendations from the public health departments has helped to contain the disease so far in Australia. The two vaccines that have been approved for use in Australia have been successful in preventing severe disease, hospitalisation and death.18,19 It is reasonable to expect that incidence of COVID-19-related renal disease would be contained by this measure. Unfortunately, the clinical vaccine trials did not include patients with renal disease. The efficacy of vaccines, in general, is poor in patients on dialysis and transplant recipients because of their poor immune response. Live vaccines are contraindicated as well. In spite of the inadequacy of available vaccine trial data, the Australian and New Zealand Society of Nephrology15 and other bodies20 have called for all patients with CKD, those on dialysis and transplant recipients to be vaccinated because of poor outcomes from the natural course of COVID-19. Further studies are needed to judge the efficacy of the vaccines in improving outcomes and survival in this vulnerable population.
Details of hospital-based interventions for AKI, as well as screening and managing patients on dialysis and transplant recipients, are not addressed as they are beyond the scope of this review.
Conclusion
Our understanding of COVID-19-related renal disease and the effect of the infection in patients with renal disease is still evolving. The evidence currently available indicates that COVID-19 appears to cause significantly poorer outcomes in patients with AKI or on dialysis and transplant recipients when compared with the general population. AKI appears to occur in individuals with severe COVID-19, and the pathogenesis is likely multifactorial. Identifying individuals at high risk for severe disease may help with prevention and management of COVID-19-related renal disease.
Key points
- COVID-19 leads to poor prognosis in patients with AKI or on dialysis and transplant recipients, with high mortality rates reported. Recovery from AKI is often incomplete in survivors.
- AKI is more common in individuals with severe COVID-19 disease.
- Identifying individuals at high risk for severe disease (eg older age group, obesity, diabetes, cardiovascular and cerebrovascular disease and immunosuppression), instituting measures to avoid dehydration and avoiding nephrotoxic agents (especially radiocontrast dyes), among other measures, will help with prevention and management of COVID-19-related renal disease.
- Australia has been spared a heavy onslaught from COVID-19 when compared with the other nations as a result of the vigilant public health units in the various states of the Commonwealth.
- In the absence of any effective direct antiviral therapy, prevention from successful public health measures and effective vaccination is the cornerstone of management.