It has been five years since medicinal cannabis was legalised in Australia, and we are now seeing a rapid escalation in the use of medicinal cannabis products within legal pathways.1 The aim of this review is to provide a primer on the safety issues that need to be considered with medicinal cannabis. Historically, the toxicology of cannabis has been viewed within the prism of its recreational use, where it was once deemed a very dangerous and highly addictive drug. However, recently the United Nations accepted recommendations of the World Health Organization (WHO) to remove cannabis from Schedule IV of the Single Convention on Narcotic Drugs, in recognition of the fact that cannabis has legitimate medicinal properties and a more acceptable safety profile than previously thought.2–5
What is medicinal cannabis?
Medicinal cannabis is not just a single entity and encompasses a diversity of products.1 Cannabis contains approximately 500 molecules, including approximately 100 plant-derived cannabis compounds (phytocannabinoids), terpenes and flavonoids. The best-characterised phytocannabinoids are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC is responsible for the intoxicating effects of recreational cannabis, whereas CBD is not intoxicating.6
Many products contain different ratios of CBD and THC, for example 10:1, 20:1 or 50:1 (Figure 1). Some products will contain CBD or THC alone as highly purified active pharmaceutical ingredient (API)–containing formulations that are often referred to as isolates. These formulations do not contain other cannabinoids, terpenes or flavonoids. Other products contain CBD and/or THC with a ‘full-spectrum’ of cannabis plant constituents including other phytocannabinoids (eg cannabichromene, cannabigerol, Δ9-tetrahydrocannabinolic acid or cannabidiolic acid) as well as terpenes and flavonoids, all of which may have therapeutic effects.7–10 To ascertain exactly what is contained in a given medicinal cannabis product, a request can be made to the manufacturer for a certificate of analysis. Therapeutic daily doses of CBD are typically between 50 mg and 1500 mg, which are greater than those for THC, which are between 5 mg and 20 mg.1
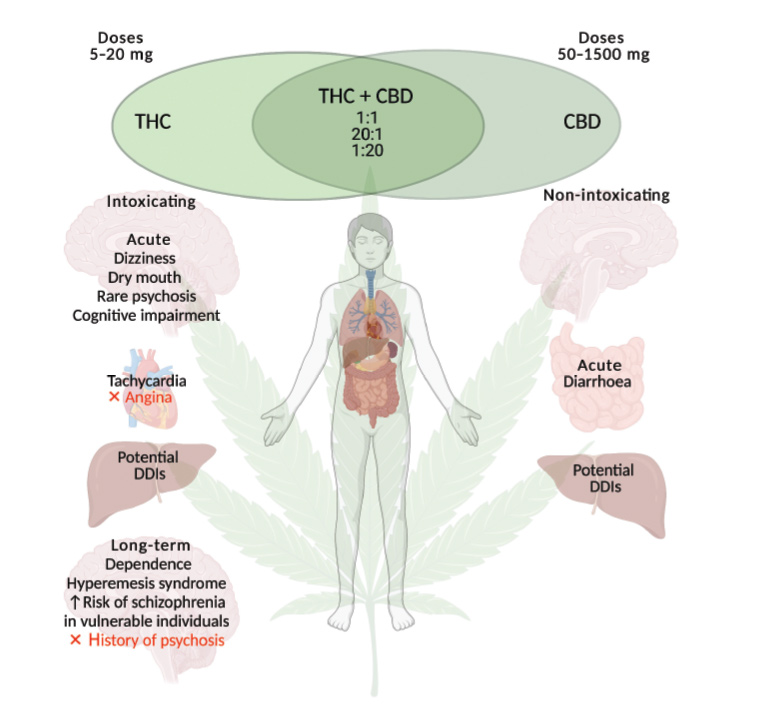
Figure 1. The acute and long-term adverse effects of medicinal cannabis. Medicinal cannabis is generally well tolerated when appropriate doses are used. The products contain Δ9-tetrahydrocannabinol (THC) and/or cannabidiol (CBD). There are more safety concerns regarding THC than CBD. Medicinal cannabis containing THC is contraindicated in patients with angina or a history of myocardial infarction, or a personal or family history of schizophrenia or psychotic-related disorders. Vigilance is required when medicinal cannabis is prescribed to patients taking conventional medications because of the potential for drug–drug interactions (DDIs).
Figure created with Biorender.com
When evaluating the safety profile of medicinal cannabis products, it is important to consider the relative THC and CBD content, as CBD generally has fewer safety concerns than THC. Indeed, on the basis of CBD’s excellent safety profile,11 the Therapeutic Goods Administration (TGA) recently approved a legal pathway for low-dose CBD formulations to be provided over the counter as Schedule 3 pharmacist-only medications for conditions that do not require medical oversight. However, no product has yet been formally approved within the pathway, and the onus is on companies to provide scientific data to the TGA to support registration.
Approved products
The only cannabis-based medicines that are registered by the TGA are nabiximols (Sativex) and CBD (Epidyolex). Sativex is an oromucosal spray formulation containing equal doses of both THC and CBD and is indicated for treating spasticity associated with multiple sclerosis. Sativex contains two cannabis plant extracts in peppermint oil corresponding to 27 mg/mL THC and 25 mg/mL CBD. Epidyolex is indicated to treat intractable childhood epilepsies and is taken orally via syringe. Epidyolex was recently listed on the Pharmaceutical Benefits Scheme (PBS) and its cost subsidised by the Australian Government. Epidyolex contains 100 mg CBD/mL of sesame oil and is devoid of THC. Extensive preclinical and clinical toxicological data were collected on these approved cannabis-based medicines and were included in their registration dossiers. The nabiximols gives rise to acute adverse effects indistinguishable from THC alone.3,4
Unapproved products
The rest of the cannabis-based medicines on the market are available as ‘unapproved’ products via the Special Access or Authorised Prescriber Schemes.1 As at 2 March 2021, the TGA has approved 99,000 applications via Special Access Scheme (SAS) category B for patients to access unapproved medicinal cannabis products. There are approximately 150 unapproved medicinal cannabis products on the market that must abide by the Australian standard for medicinal cannabis (TGO 93), which aims to ensure the products have reliable cannabinoid content and are free of toxic contaminants. CBD-dominant products (³98% pure CBD) are Schedule 4, prescription-only medications when prescribed to patients with conditions that require medical oversight. In contrast, products containing THC are Schedule 8 controlled medications, meaning prescriptions require state or territory health department approvals as THC is classified as a drug of dependence.1
Acute adverse effects
Cannabis containing THC
Cannabis is a relatively safe drug, and it is not associated with fatal overdoses. On the basis of animal lethal dosing studies, the human lethal dose of THC has been extrapolated to be >15,000 mg.12 This highlights that THC has a very wide safety margin, as this lethal dose is 750 times greater than a typical intoxicating dose of 20 mg. Unlike the opioids, cannabis does not cause respiratory depression because of a paucity of cannabinoid receptor expression in the brainstem.
A major issue with acute dosing of medicinal cannabis products that contain THC is intoxication. A typical intoxicating dose of THC in a person who has never used cannabis previously is approximately 10 mg, although caution is warranted as some patients may be more sensitive.1 When intoxicated, patients may experience euphoria and anxiolysis as well as enhanced sensory perceptions. Higher doses of THC are associated with anxiety, panic and disorientation in some individuals. Subtle cognitive deficits such as impaired attention and short-term memory impairment may be experienced.13 THC-containing cannabis may impair driving performance.14 THC in medicinal cannabis may also cause the classic ‘munchies’ effect by enhancing appetite. Dry mouth and dizziness are common side effects. Cannabis may also cause nausea and vomiting.15
Cannabis may provoke a transient psychosis in some healthy participants because of its THC content,16,17 although these reactions are rare. In a study that examined emergency hospital admissions across 14 European countries over a six-month period, only seven cases of cannabis-induced psychosis were found when cannabis was the only drug used.18 CBD-dominant cannabis products may also contain THC, and so it is important that doctors consider the THC content in their dosing recommendations to avoid THC-related intoxication.
Cannabis containing CBD
CBD administered as a highly purified substance is very safe and not intoxicating. Phase 1 studies demonstrate that it is well tolerated even at very high doses (up to 6000 mg).19 The most common side effect of CBD is diarrhoea.20 The main area of concern related to CBD’s safety is the potential for drug–drug interactions that might occur when CBD is administered with conventional pharmacotherapies. CBD is an inhibitor of cytochrome P450 (CYP450) enzymes such as CYP3A4 and CYP2C19, and high doses of CBD used to treat childhood epilepsies increase plasma concentrations of anticonvulsant medications, particularly clobazam, which can lead to increased sedation.21–23 Co-administration of CBD with valproate is suspected to cause an elevation of liver transaminases. CBD has also been observed to cause clinically significant pharmacokinetic interactions with warfarin, tacrolimas and methadone by magnifying the adverse effects of these medications.24–27 Accordingly, careful upwards dose titration of CBD-dominant products is best practice, especially in patients taking other medications. It is important that doctors carefully monitor patients for any side effects of their concomitant medications when commencing medicinal cannabis products.
Effects on the cardiovascular system
It would be prudent to advise against the use of medicinal cannabis containing THC in patients with angina or a history of myocardial infarction. Cannabis containing THC can cause tachycardia, and there are reports of cannabis-induced myocardial infarction.28 A meta-analysis of studies reported that acute THC increases heart rate by eight beats per minute, and that the effects are dose dependent.29 With repeated dosing, the tachycardic effects diminish because of tolerance. Interestingly, some studies in healthy participants and patients with hypertension showed longer-term exposure to THC or cannabis reduced blood pressure.30,31 Acute or repeated dosing with CBD does not appear to affect heart rate or blood pressure.32
Long-term adverse effects
There is a need for more safety and pharmacovigilance studies with medicinal cannabis products. Most evidence on the potential long-term adverse effects of cannabis derives from its recreational use, which may not readily apply to medicinal cannabis use. Recreational cannabis is unregulated and contains high concentrations of THC, which is exploited for the specific purpose of intoxication.33 However, medicinal cannabis is regulated by specific manufacturing standards and is prescribed under medical supervision using strict dosing regimens. Further, studies on the adverse effects of recreational cannabis involved smoking as the dominant route of administration, which is not recommended in a medicinal cannabis setting. Cannabis smoking also encompasses mixing cannabis flower with tobacco, which further complicates interpretation of studies on the harms of recreational cannabis.34 It must also be recognised that most of the evidence on the adverse effects of recreational cannabis use relies on observational, population-based studies, which may include multiple confounders and cannot unequivocally infer causation. All these limitations must be kept in mind when evaluating the existing evidence. In time there will be better evidence for any long-term health impacts of medicinal cannabis more specifically, which is likely to be safer than recreational cannabis.
An excellent evaluation of the long-term toxicity of THC comes from a placebo-controlled randomised controlled trial (RCT) in 329 patients with multiple sclerosis in which THC was administered daily to participants for three years (up to 28 mg/day).35 This trial concluded that THC has an acceptable safety profile with low-to-moderate toxicity and a low incidence of serious adverse events; there was no difference in the total number of adverse events in the placebo group versus the THC group.35 The most common adverse effects of THC when compared with placebo were dizziness and light-headedness, and dissociative thinking or perception disorders. More research is needed to address the long-term effects of CBD. Long-term adverse effects of CBD were reported in patients with childhood epilepsy receiving Epidyolex under an expanded access program for up to 144 weeks.36 The adverse effects were similar to those reported in shorter-term trials, with the most common adverse effects being somnolence (30%) and diarrhoea (24%).
Cognitive function
Acute use of cannabis containing THC may impair cognitive and memory function, especially when intoxicating doses of THC are administered. However, evidence for long-term and enduring cognitive and memory dysfunction is highly contentious. A systematic review and meta-analysis of 69 cross-sectional studies in a recreational context found only a small effect size for reduced cognitive functioning in people who used cannabis frequently at high doses for an extended period.37 The study’s authors questioned the clinical significance of the cognitive impairments for most people who use cannabis. There was no association between cannabis use and reduced cognitive function in studies in which the participants were drug-free for >72 hours, thus suggesting any cognitive impairing effects were reversible.
Some small-scale studies have reported structural abnormalities in the brains of people who used cannabis heavily over a long period of time in a recreational setting.38–40 However, these effects were not replicated in larger studies that controlled for confounding variables (eg alcohol use, tobacco use).41–43 A high-profile article reported that long-term cannabis use commencing in adolescence reduced IQ.44 However, there were no reductions in IQ in those who commenced use in adulthood and had been abstinent for a year, suggesting adult use does not have any residual effects on cognitive function. Notably, the same authors more recently revised their conclusions, stating that adolescent cannabis use was not responsible for the reductions in IQ, but rather it was explained by familial factors.45–47
In conclusion, the prescription of medicinal cannabis for adults is unlikely to have irreversible adverse effects on cognitive function. Patients should be monitored for acute impairing effects on cognition that might arise with medicinal cannabis containing THC. The effects of CBD products that do not contain THC would be of less concern, as CBD does not appear to affect cognitive function.48
Drug dependence
Repeated recreational use of street cannabis for the sole purpose of intoxication may lead to cannabis dependence. People who use cannabis recreationally may seek out professional treatment to assist with drug abstinence. Thirteen per cent of people who have used cannabis as a recreational drug will become cannabis dependent.49 Individuals with cannabis dependence crave the drug, lose control of their drug use and use cannabis for longer periods than intended. Individuals with dependence may also experience a cannabis withdrawal syndrome when they abstain from cannabis use, although this is mild when compared with syndromes associated with alcohol, benzodiazepines or heroin. Cannabis withdrawal promotes symptoms of insomnia, depression, anxiety and gastrointestinal disturbance that may last 48–72 hours.50 Overall, cannabis has mild-to-moderate addictive liability. The issue of cannabis dependence in those using it purely for medicinal purposes has yet to be adequately studied. It may be that medicinal use of cannabis, where intoxication is not the primary aim, may be less susceptible to any habit-forming effects of THC. CBD is not habit-forming.51
Psychosis and psychosis-related disorders
Acute cannabis-induced psychosis has been documented but is rare. Of more concern is the link between cannabis use and schizophrenia. Human population studies suggest that there is a dose-dependent increase in the risk of developing schizophrenia in those who commence cannabis use during adolescence as a recreational drug.52,53 Some argue against the view that cannabis causes schizophrenia by citing that the rapid increase in global cannabis use in recent decades has not translated into an increased incidence of schizophrenia.54–56 Most agree that cannabis does not directly cause schizophrenia, but rather cannabis may be permissive to schizophrenia in individuals who are already vulnerable.
It must be emphasised that the vast majority of people who use cannabis will never develop a psychotic disorder. It has been estimated that 4700 young people would need to be dissuaded from cannabis use to prevent a single case of schizophrenia.57 In any case, doctors should not prescribe medicinal cannabis with THC content to patients who have a known sensitivity to cannabis or to those who have a personal or family history of schizophrenia or psychotic disorders. Interestingly, purified CBD has antipsychotic properties in humans.58
Cannabis hyperemesis syndrome
There are rare case reports of long-term, heavy recreational cannabis use promoting severe nausea and vomiting, which is known as the cannabis hyperemesis syndrome.59–61 The syndrome is associated with compulsive bathing behaviour and resolves on cessation of cannabis use. Cannabis hyperemesis syndrome appears to be mediated by THC, and there is no evidence of CBD causing the syndrome. There have been no reports of cannabis hyperemesis occurring in patients taking medicinal cannabis, which may be due to lower doses of THC being prescribed.
Conclusion
Medicinal cannabis is generally well tolerated, but the science related to its potential adverse effects is in its infancy. What has been learnt from the adverse effects of recreational cannabis use may not readily apply to medicinal cannabis, which is administered under medical supervision using strict dosing regimens and non-smoking routes of administration. High-quality safety studies are needed, especially of unapproved medicinal cannabis products, which are increasingly being used in the community. New, unforeseen adverse events may arise in this rapidly evolving area of medicine, and pharmacovigilance measures must be implemented to ensure patient safety.
Key points
- Medicinal cannabis is generally well tolerated when prescribed at appropriate doses; CBD has fewer safety concerns than THC.
- It is recommended that patients are prescribed lower doses, which are slowly up-titrated (beginning with THC doses <10 mg).
- For patients taking concomitant medications, it is important to carefully monitor for dose-related side effects and consider therapeutic blood monitoring for drugs with a narrow therapeutic window.
- Medicinal cannabis with THC content should not be prescribed to patients with angina or a history of myocardial infarction, or to those with a personal or family history of schizophrenia or psychotic disorders.
- Unless the benefits clearly outweigh the risks, medicinal cannabis should not be prescribed during pregnancy, nor should medicinal cannabis with THC content be prescribed to children or adolescent patients.