Legal access to medical cannabis is increasingly common in Australia, with the Therapeutic Goods Administration (TGA) having approved >100,000 Special Access Scheme Category B applications for patient access to cannabinoid products as of March 2021.1 It is important for physicians and their patients to understand the driving-related risks that medical cannabis use may confer.
The aim of this article is to briefly review the scientific evidence around cannabis and driving impairment and discuss current legal issues affecting patients, as well as to update physicians on relevant issues and the best guidance to offer their patients.
The two major cannabinoids: THC and CBD
Of the hundreds of bioactive molecules in the cannabis plant, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most abundant and the best characterised. THC is responsible for the intoxicating effects of cannabis, and current evidence supports its efficacy in treating chronic pain, chemotherapy-induced nausea and vomiting, and spasticity in multiple sclerosis.2–4
In contrast, CBD is non-intoxicating and has current regulatory approval in Australia for certain rare forms of childhood epilepsy as a 100 mg/mL oral formulation (Epidyolex). There is also emerging evidence of CBD efficacy in treating anxiety, psychosis, chronic pain and neurological disorders.5–7 There is some limited evidence that CBD can counteract negative THC-related side effects such as anxiety and paranoia.8,9 There is a rapidly growing worldwide market for CBD ‘wellness’ products. Such products typically contain very low CBD doses (eg 5–50 mg) that are of uncertain therapeutic value.10,11
With the exception of the oral THC/CBD spray nabiximols (Sativex) and the CBD oil Epidyolex, medical cannabis products in Australia are all unregistered medicines as they have not been formally assessed by the TGA for safety, quality or efficacy. These are predominantly oral preparations, oromucosal sprays and capsules; they can be THC dominant, CBD dominant or contain a specific mix of THC and CBD.12 Cannabis plant material is also available but less commonly prescribed. Therapeutic THC doses are typically in the range of 5–20 mg, while CBD doses tend to be higher (eg 50–1500 mg).
The TGA has recently announced that CBD products meeting certain specifications will be down-scheduled from Schedule 4 to Schedule 3 and therefore available in pharmacies as over-the-counter products. Such products will be limited to a maximum dose of 150 mg/day and for oral or sublingual administration. At present, there are no CBD products that have obtained regulatory approval under Schedule 3,13 although there likely will be within the next 12–24 months once companies have had products approved by the TGA for registration on the Australian Register of Therapeutic Goods.
Cannabis pharmacokinetics
Smoking or vaporising cannabis produces a rapid and transient peak in blood and oral fluid THC concentrations. When taken orally, cannabis is absorbed more slowly through the gastrointestinal tract, producing far lower blood THC concentrations. THC is highly lipophilic and is readily absorbed into fatty tissue, from where it can slowly re-enter the bloodstream days or even weeks following cannabis consumption. Blood THC concentrations are therefore not necessarily indicative of recent cannabis consumption or the amount of cannabis consumed. The mere presence of THC in blood or oral fluid THC does not reliably predict impairment, although current mobile drug testing methods and associated laws rely entirely on this.14
Cannabis and crash risk: Evidence from epidemiological studies
Epidemiological studies aim to quantify the impact substances have on road safety by estimating relative crash risk. This is an odds ratio describing the likelihood of a driver who tests positive for a drug or alcohol being involved in a crash relative to a sober driver.
The most recent and authoritative meta-analyses in this field suggest that cannabis-positive drivers are approximately 1.1–1.4 times more likely to be involved in a crash than sober drivers15,16 and are also more likely to be culpable for a crash.17 Notably, however, there have also been major recent studies in which no increases in crash or culpability risk were detected,18 particularly when drivers had low blood THC concentrations (<5 ng/mL).19 Overall, the increase in crash risk associated with THC is similar to that associated with a low-range blood alcohol concentration (BAC; 0.01–0.05 g/L),20 although some analyses suggest that crash risk and culpability with cannabis may be greater with higher blood THC concentrations.17,19 Table 1 shows relative crash risk and culpability estimates for cannabis, alcohol and other drug classes.
| Table 1. Crash risk and crash culpability estimates for different drug classes |
| Drug class |
Crash risk estimate |
Crash culpability estimate |
| Alcohol (BAC = 0.02) |
1.03–1.1918,46 |
1.3618 |
| Alcohol (BAC = 0.05) |
1.38–1.7518,46 |
2.1918 |
| Alcohol (BAC = 0.08) |
2.69–2.9218,46 |
3.6318 |
| Cannabis |
1.11–1.4215,16,47–49 |
1.20–1.4215,16,47 |
| Antidepressants |
1.35–1.4048,50 |
N/A |
| Antihistamines |
1.1248 |
N/A |
| Benzodiazepines and Z-hypnotics |
1.17–2.3048,51 |
1.4151 |
| Opiates |
1.68–2.2948,52 |
1.4752 |
| BAC, blood alcohol concentration; N/A, not available |
Cannabis and driving: Evidence from experimental studies
Experimental driving studies typically administer drugs to volunteers and then examine effects on driving using a driving simulator or, less frequently, during real-world highway driving (called on-road studies). The most common outcome measure in these studies is the standard deviation of lateral position (SDLP), a measure of lane weaving (Figure 1) that is highly sensitive to the effects of alcohol and other sedative drugs and indicates reduced vehicular control.21,22
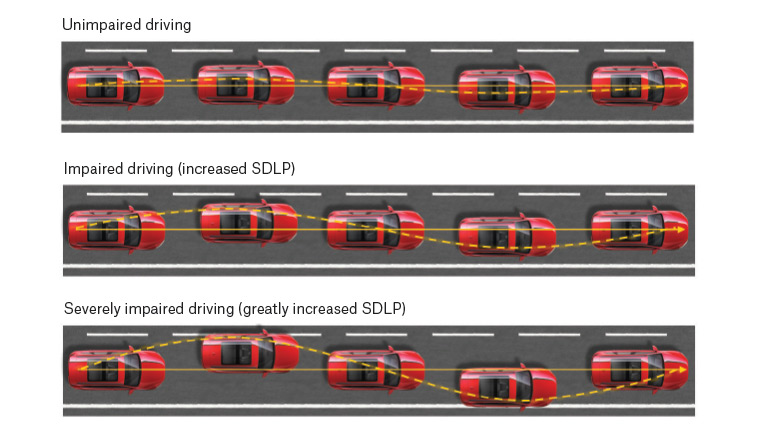
Figure 1. The standard deviation of lateral position (SDLP) is a commonly used measure of the variance in the lateral position of a vehicle within a lane. The lower panels show increased SDLP, indicating greater loss of vehicular control, or increased ‘weaving’.
Driving simulator studies have shown that cannabis increases SDLP in a dose-dependent manner. Other effects of cannabis, such as reduced speed and increased headway (distance to the car in front), are observed in some, but not all, studies.23–25 These may be compensatory effects that intoxicated drivers use when recognising their own impairment.
On-road driving studies are more ecologically valid and therefore generate higher-quality evidence than driving simulator studies. These studies indicate that cannabis-induced increases in SDLP are of a similar magnitude to low-range BACs (approximately 0.05 g/L),22,26 10 mg diazepam27 or one night of sleep deprivation.28 A recent study involving some of the current authors investigated on-road driving performance in people who use cannabis occasionally who received 13.75 mg THC (within the range of typical therapeutic THC doses in Australia [5–20 mg]10).9 Results confirmed modest but clinically relevant driving impairment at 40–100 minutes but not 240–300 minutes post-treatment (Figure 2).These findings are the first to indicate that moderate doses of inhaled cannabis are unlikely to impair driving performance for more than four hours.
![Figure 2. Mean (standard error of the mean [SEM]) change in standard deviation of lateral position (SDLP) from placebo during on-road highway driving tests following vaporisation of cannabidiol-dominant cannabis (CBD), Δ9-tetrahydrocannabinol-dominant cannabis (THC) and THC/CBD-equivalent cannabis (THC/CBD)](/getattachment/AJGP/2021/June/Medical-cannabis-and-driving/AJGP-06-2021-Focus-Arkell-Medical-Cannabis-and-Driving-Fig-2.jpg.aspx?lang=en-AU)
Figure 2. Mean (standard error of the mean [SEM]) change in standard deviation of lateral position (SDLP) from placebo during on-road highway driving tests following vaporisation of cannabidiol-dominant cannabis (CBD), Δ9-tetrahydrocannabinol-dominant cannabis (THC) and THC/CBD-equivalent cannabis (THC/CBD). The dotted line represents the SDLP increase associated with a blood alcohol concentration (BAC) of 0.02 g/L, and the solid line represents the SDLP increase associated with a BAC of 0.05 g/L.
BAC, blood alcohol concentration; CBD, cannabidiol; SDLP, standard deviation of lateral position; THC, Δ9-tetrahydrocannabinol
Reproduced with permission from Arkell TR, Vinckenbosch F, Kevin RC, Theunissen EL, McGregor IS, Ramaekers JG, Effect of cannabidiol and Δ9-tetrahydrocannabinol on driving performance: A randomized clinical trial, JAMA 2020;324(21):2177–86, doi: 10.1001/jama.2020.21218.
When given together with THC in a 1:1 ratio, CBD does not appear to attenuate THC-induced driving or cognitive impairment.9,23 CBD itself (13.75 mg) did not impair driving in a recent on-road study when given alone in the form of vaporised CBD-dominant cannabis.9 Another randomised controlled trial investigating the dose-dependent effects of oral CBD (15 mg, 300 mg and 1500 mg) on driving is currently nearing completion in these authors’ laboratory.29
A recent meta-regression analysis by the current authors surveyed all relevant articles from the past 20 years that assessed THC-induced impairment of driving-related cognitive and psychomotor function. This analysis concluded that most driving-related cognitive skills recover within approximately five hours of inhaling 20 mg THC in individuals who use cannabis occasionally, although impairment of some specific skills may take longer (<7 hours).30 Impairment may also be extended with higher THC doses and with oral formulations. Figure 3 summarises predicted impairment recovery times with 10 mg and 20 mg THC doses using inhaled and oral routes of administration.30 While there are no uniform dosing standards for cannabis, 20 mg THC is a relatively high acute dose and is the upper limit of typical therapeutic doses in Australia. A 10 mg THC dose might be considered a typical acute dose and is equivalent to approximately four sprays of nabiximols (Sativex, an oromucosal spray containing a 1:1 ratio of THC and CBD that is widely used for the treatment of spasticity in multiple sclerosis, with each spray containing 2.7 mg THC).
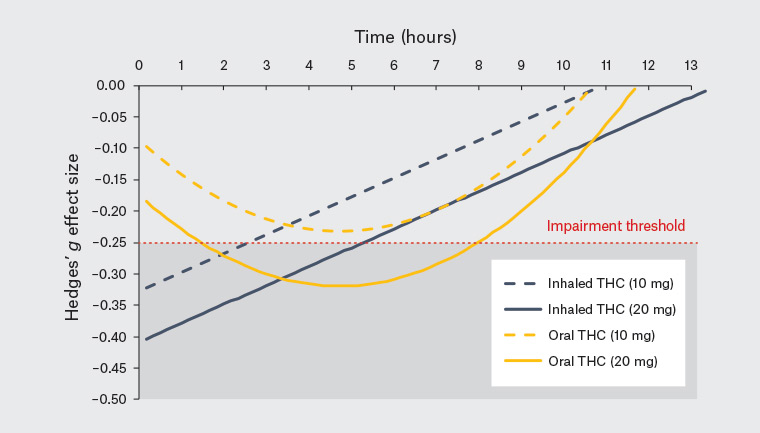
Figure 3. The predicted effect size (Hedges’ g) of Δ9-tetrahydrocannabinol (THC) on driving and driving-related cognitive skills (with all ‘skills’ aggregated) in people who use cannabis occasionally over time as reported in McCartney et al.30 Negative effect sizes indicate an impairing effect of THC. Separate lines represent orally administered (yellow) and inhaled (blue) THC at doses of 10 mg (dashed) and 20 mg (solid). The red line represents a Hedges’ g effect of –0.25 (the limit at which minimal impairment remains). Overall, these data show that performance tends to recover at five hours following higher doses of inhaled THC and at eight hours following higher doses of oral THC.
In a recent survey of Australians who use medical cannabis, most respondents (72%) believed that their medical cannabis use does not impair their driving.31 A similar percentage (71%) reported that their medical cannabis use does not affect their ability to assess their fitness to drive. Just over one-third (35%) of respondents reported typically driving within three hours of cannabis use. These findings highlight a need for patient education regarding the risks associated with driving under the influence of cannabis. In particular, it is important for patients to understand that THC can still impair their driving even if they do not feel intoxicated.
Medical cannabis and driving
The vast majority of cannabis and driving studies have focused on non-medical cannabis use and have involved young, healthy participants who use cannabis occasionally and are given THC doses causing robust intoxicating effects (eg feeling ‘stoned’). Rigorous experimental studies on the effects of medical cannabis treatment on driving performance in patients are urgently needed to better guide policy in this area.
Some relevant data come from a recent review of patients undergoing long-term treatment with nabiximols. The majority of these patients reported either no change or an improvement in their self-reported driving ability, possibly due to reduced spasticity and/or improved cognitive function.32 Most patients also showed a slight improvement in fitness to drive as assessed using a computerised test battery following 4–6 weeks of treatment.33 Similarly, another recent review reported that THC rarely impairs driving-related cognitive skills (eg inhibitory control, information processing, reaction time, sustained attention) in clinical populations (eg diabetic neuropathy, Tourette syndrome, attention deficit hyperactivity disorder, dementia); that is, only one of the six studies reviewed detected a significant impairing effect of THC.30
As medical cannabis patients typically use cannabis products daily and over prolonged intervals, they will likely develop behavioural and pharmacological tolerance to THC effects that may mitigate driving impairment. One key study reported that participants who heavily use cannabis (ie cannabis use on a daily or near-daily basis) showed no driving impairment with either 10 mg or 20 mg dronabinol (synthetic THC), while those who use cannabis occasionally (ie cannabis use <1 time per week) showed the expected impairment, particularly at the higher 20 mg dose.34 This implies that driving impairment is likely to be greatest in the early stages of THC treatment. Doses of THC should be titrated slowly upwards in patients during the first few weeks of initiation, and patients should be advised to exercise extreme caution around driving until their treatment regimen is stable.
Mobile drug testing
All Australian jurisdictions carry out random mobile drug testing (MDT), analogous to random breath testing for alcohol. This is a three-stage process involving an initial and a secondary oral fluid test at the roadside using two different devices. If both roadside tests are positive, oral fluid is then subject to confirmatory analysis in government analytical laboratories. The three drugs that are usually tested for are THC, methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA), with cocaine also tested for in NSW. It is important to note that MDT only tests for the presence of drugs and not for impairment, and that driving with the presence of an illicit drug (ie ‘mere presence’) is a separate offence from driving under the influence.
THC enters oral fluid when cannabis products are smoked, vaporised or eaten through contamination of the oral cavity. There is no evidence that THC can be transferred from blood into oral fluid, meaning that products that avoid THC deposition in the oral cavity (eg THC capsules, patches or suppositories) are unlikely to give rise to a positive roadside drug test. There are no current legal prohibitions related to driving in patients using CBD-only products, and there is no evidence that CBD can give rise to positive roadside drug tests in the absence of THC.
Legal medical cannabis is not a valid defence against prosecution under MDT laws, and patients face potentially severe penalties for driving with legal THC products in their system. This is a major barrier for patients contemplating or receiving medical cannabis treatment, particularly patients who live in regional and remote areas who depend on being able to drive for their employment and quality of life.
Legislative changes
The Victorian government is currently considering legislation that would allow patients using medical cannabis to legally drive with THC in their systems as long as they are not impaired.35 This would bring cannabis and driving laws insofar as they apply to patients into line with current laws for other drugs known to impair driving, such as opioids and benzodiazepines. These changes would not extend to the large number of patients self-medicating with illicit cannabis products.36
THC detection times
THC is typically detectable in oral fluid for 4–6 hours after smoking or vaporising cannabis, although this is highly variable across individuals and depends on factors such as salivary composition, flow rate, time since eating and frequency of THC use. In very heavy cannabis users, THC may be detectable in oral fluid for up to three days following abstinence.37,38 In blood, THC is commonly detectable for up to seven days,39,40 and in extreme cases, for up to 30 days41 following cessation of use. In urine, THC may be detectable for up to 24 days, depending on the sensitivity of the test.42 The secondary, inactive metabolite of THC, 11-nor-9-carboxy-THC (THC-COOH), which is very commonly used in workplace drug tests, can be detected for up to three months in very heavy cannabis users.43
International approaches to detecting cannabis-impaired driving
Australia is the only country with a widespread random MDT program for detection of THC. Other jurisdictions (eg the Netherlands, Belgium, France) have legal limits for THC in oral fluid but typically only request samples when there is evidence of impaired driving. In Canada, where cannabis use was fully legalised in 2018, oral fluid tests such as those used in Australia can be used to confirm a suspected case of drug-impaired driving, but only when an officer can first demonstrate impaired driving.
Other jurisdictions, including various states within the USA, rely on field sobriety tests to assess driving impairment (eg walk and turn test, finger to nose test, one-legged stand). These tests have good sensitivity to alcohol intoxication but limited sensitivity to cannabis intoxication.44
Other factors that may affect driving
Combining cannabis with alcohol produces additive effects that can lead to driving impairment of greater severity.25 Patients should be particularly cautious around their use of alcohol and other sedative drugs when also using medical cannabis. CBD appears unlikely to exacerbate alcohol-induced driving impairment, although this interaction has yet to be studied.
CBD is a potent inhibitor of certain cytochrome P450 enzymes (eg CYP3A4, CYP2C19, CYP2C9), which play a key part in drug metabolism.45 Pharmacists and physicians should therefore consider possible drug interactions in patients using CBD products alongside prescription drugs that are substrates of these enzymes and that have sedative or intoxicating properties in their own right (eg some benzodiazepines, antipsychotics and anticonvulsants).
Conclusion
Driving is a complex task involving a range of cognitive and psychomotor functions. Any substance that interferes with these functions can be deleterious for driving. The effects of THC on driving are generally modest and appear similar to the effects of low-dose alcohol. However, impairment may be more pronounced and potentially severe in patients who are cannabis-naive or where cannabis is combined with alcohol or other impairing drugs. Patients using THC-containing products should be advised to avoid driving and other safety-sensitive tasks (eg operating machinery) during the initiation of treatment with THC-containing medicinal cannabis products and in the hours immediately following each dose. Patients using THC-containing preparations are also at risk of testing positive for cannabis in oral fluid even if they are not impaired. CBD-only medications appear to pose no traffic safety risk, although CBD is unlikely to ameliorate THC-induced impairment. Up-to-date information regarding cannabis and driving laws can be found on state government websites.