Dry eye disease (DED), also known as ‘keratoconjunctivitis sicca’, is a common disorder that negatively affects the quality of life of hundreds of millions of people around the world.1,2 In the USA, the average cost of managing DED is estimated to be $55.4 billion.3 DED, in most cases, is not curable and involves mainly symptomatic management, even when ophthalmologist referral is indicated. It is one of the most frequent causes of visits to the optician or ophthalmologist.4 However, general practitioners (GPs) are in an excellent position to confidently assess and manage this often underdiagnosed condition.5,6
In 2017, the Tear Film and Ocular Surface (TFOS) Society established a TFOS DEWS II definition, expanding on the prior definition.1 The executive summary stated:
Dry eye is a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film and accompanied by ocular symptoms in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.1
One study showed the prevalence of DED is approximately 7.4% in Australia in the adult population,7 and the Blue Mountains Eye Study (reported in 2003) showed that 57.1% of the older population had one DED symptom.8 This is likely to be much higher now, with a significant increase in prevalence in older patients and the presumption that DED is underdiagnosed.
Anatomy and physiology
The ocular surface is composed of an epithelium layer lining the cornea, anterior globe and the tarsi.9 This surface forms part of the lacrimal functional unit, which also includes the lacrimal glands, eyelids and meibomian glands.10 Moisture and hydration of the ocular surface are maintained by an ordered layer of tear film that is part of a homeostatic process that aims to keep the eye lubricated.
The tear film has a volume of approximately 7–10 µL10 and drains via the inferior and superior lacrimal punctum into the lacrimal canaliculi, which subsequently flows into the superior lacrimal sac component of the nasolacrimal duct, and it finally drains into the nose. The tear film is composed of three layers, starting from superficial to deep: lipid, aqueous and mucin.4,11
Pathophysiology and subtypes of dry eye disease
Any alterations in the lacrimal functional unit can lead to DED, more so with the presence of risk factors that contribute to the development of DED (Figure 1). Usually, the constant drying of the open eye is offset by homeostatic mechanisms that regulate tear secretions and distribution.9
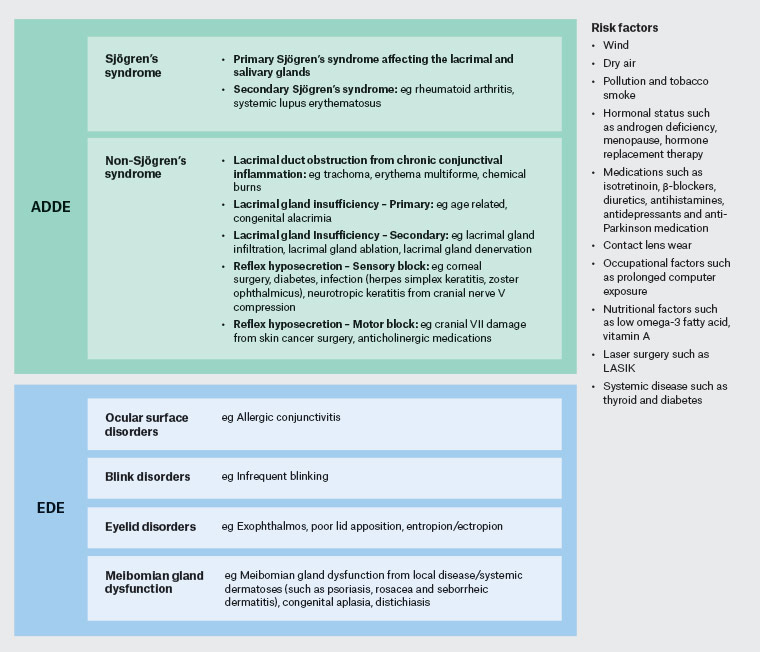
Figure 1. A diagrammatic representation of how ADDE and EDE can be divided, with associated common risk factors. Click here to enlarge
ADDE, aqueous deficient dry eye; EDE, evaporative dry eye
DED is now universally accepted as either evaporative dry eye (EDE) or aqueous deficient dry eye (ADDE).1 ADDE is as a result of increased tear film osmolarity due to hyposecretion of the lacrimal gland.9 EDE is also a result of increased tear film osmolarity, which occurs as a result of excessive water evaporation with normal lacrimal secretory function.12 The result of these processes is a hyperosmotic and desiccating environment that stimulates innate inflammatory events1 that contribute to an impairment of ocular surface homeostasis.12
The leading cause of EDE is meibomian gland dysfunction (MGD),9 which can include inflammation, hypersecretion and abnormal excreta of the meibomian glands.1,13 ‘Blepharitis’ is a broad term referring to inflammation of the eyelid. It is thought to involve staphylococcal enzymes and toxins, causing immune-mediated damage.14 It is frequently associated with other conditions such as seborrheic dermatitis, acne and rosacea. It can anatomically be divided into anterior and posterior blepharitis, which can often co-exist.12,14 Anterior blepharitis is inflammation of the anterior eyelid margin involving the eyelid skin and lashes.9 It can cause burning and grittiness in both eyes. Posterior blepharitis is often referred to as MGD that is commonly associated with rosacea.14 Signs include redness of eyelid margin with blocked meibomian glands and a frothy discharge along the eyelid margins.14
While the categories of DED exist, often distinguishing between them can be difficult due to an overlap of the mechanisms involved (eg in Sjögren’s syndrome). While it is recognised as ADDE, the effect on the meibomian gland can lead to EDE as well.1,5 Figure 2 provides a more detailed overview of the different conditions associated with DED.
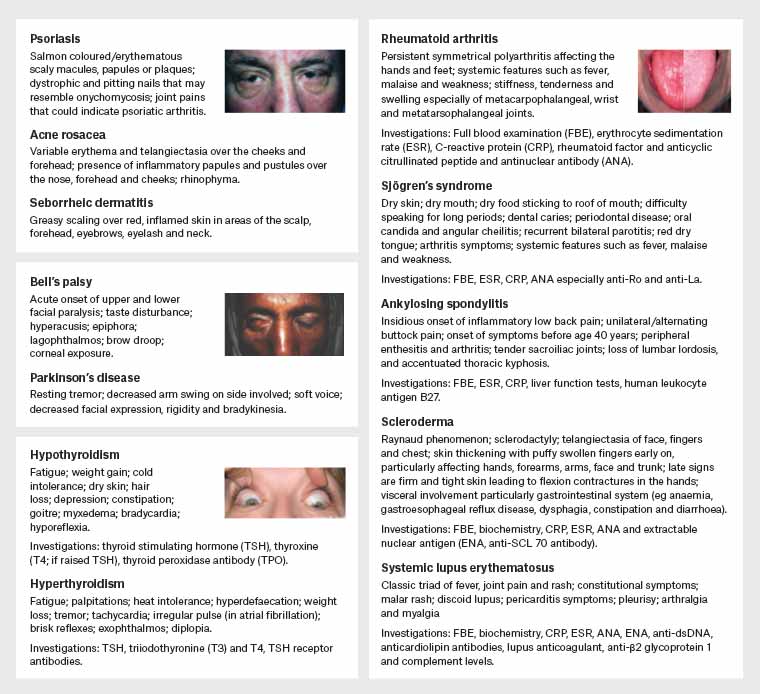
Figure 2. Overview of systemic conditions that can be associated with dry eye disease. Click here to enlarge
Images reproduced with permission from EyeRounds.org University of Iowa and Science X https://medicalxpress.com/news/2012-08-standards-diagnosis-Sjögren’s-syndrome.html
History
Diagnosis begins with a thorough history, which gathers symptoms, severity and risk factors. It is essential to ask about the symptoms, duration, exacerbating factors and ocular history.15 The common symptoms of DED are detailed in Box 1.9 Symptoms tend to be worse on waking16 and can be exacerbated by factors that are listed in Figure 2.
| Box 1. Symptoms of dry eye disease |
- Dry and gritty sensation
- Foreign body sensation
- Soreness in the eye
- Stinging or burning
- Photophobia
- Intermittent blurry vision
- Excessive tearing (due to reflex tear from corneal irritation)
- Eye fatigue
- Fluctuating diurnal vision
- Soreness
- Itching (consider allergic conjunctivitis)
|
Scores of symptom severity can be assessed with questionnaires, such as the five-item dry-eye questionnaire (DEQ-5),17 or the ocular surface disease index (OSDI).16 The DEQ-5 elicits the presence of dry eye symptoms, their frequency, severity and the time of day when they are most severe.17 A positive result for DEQ-5 is a score of ≥6.
The OSDI is a 12-item questionnaire used to assess the symptoms of ocular irritation in DED and how they affect functioning related to vision. The three areas that are screened are ocular symptoms, vision-related function and environmental triggers. The OSDI is assessed on a scale of 0–100, with higher scores representing greater disability: 13–22 represents mild DED, 23–32 represents moderate DED, and ≥33 represents severe DED.16
Past medical history can guide the clinician as to the cause. Ask about corneal refractive surgery, contact lens wear and ocular surface diseases such as allergic conjunctivitis, varicella zoster, herpes infections and previous transplants, which can lead to graft versus host disease (where donor tissue sets off an overactive systemic inflammatory response, leading to the destruction of host tissue, such as in the eye).18 Facial surgery (eg in skin cancer management) can cause trigeminal nerve or facial nerve damage, which can result in neurotrophic keratitis and corneal exposure keratopathy, respectively – both of which increase the severity of DED.1
There may be underlying systemic medical conditions and dermatological disorders associated with DED. Constitutional symptoms such as fatigue, weight loss and loss of appetite may be reported. Eliciting history by a systems review can guide in determining a possible cause for DED (Figure 1).15,19 When undertaking a social history, it is good practice to ask about smoking, as this may associate with the risk of DED in the general population.19,20 Medication history is essential, as clinicians are mainly looking for risk factors that may cause dry eye, as listed in Figure 2.
Examination
The first step is to perform an external examination with a focus on skin, eyelids, adnexa, proptosis and any visible cranial nerve deficits (mainly looking for 3rd, 5th and 7th nerve deficits, which may affect lid closure and blink rate). Check for any eyelid deformity such as ectropion/entropion as well as incomplete closure and infrequent blinking. Look for any erythema, thickening of the eyelid margins and the presence of discharge as well as dandruff-like scales (indicating blepharitis; Figure 3A).5 The discharge should be a transparent liquid oil, while thick or discoloured meibum indicates dysfunction.5 Expression of the meibomian gland with gentle pressure to the lid margin from a cotton bud can determine whether the leading cause of EDE – MGD – is playing a role .1 It is also a validated way of unblocking the glands if this is an issue.
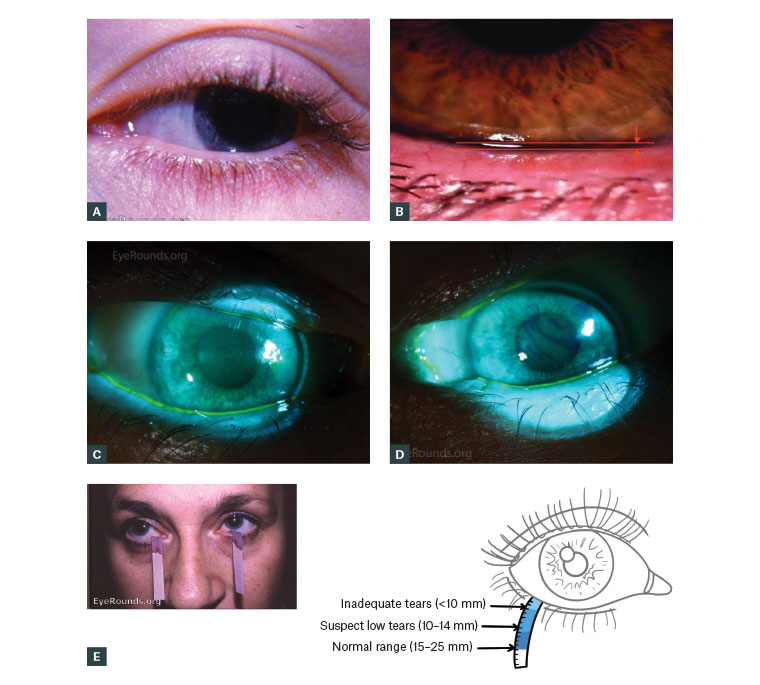
Figure 3. Images portraying information that can lead to a diagnosis of dry eye disease
A. Blepharitis of the eye; B. A normal tear film height; C,D. Tear break-up time, which is abnormal if dry spot/hole is seen at less than 10 seconds; E. The Schirmer test
Figures 3A, 3C and 3D reproduced with permission from EyeRounds.org University of Iowa
Figure 3B reproduced with permission from The College of Optometrists29
Checking visual acuity is an integral part of the examination, as worse visual acuity drives vision-related symptoms in dry eye.21 Examining the adnexa may give evidence of lacrimal gland enlargement. If there is suspected trigeminal nerve dysfunction, then corneal sensation should be assessed with a cotton wisp.
If a slit lamp is available, then biomicroscopy evaluation is useful. The focus should be on the tear film, the eyelashes, the eyelid margins, puncta, conjunctiva and cornea.12 If a slit-lamp biomicroscopy is not available, then an ophthalmoscope with a high plus lens will give a better resolution than the ophthalmoscope alone.
The normal tear film height is 0.3 mm (Figure 3B); while this is being assessed, the viscosity and presence of debris and mucous strands should be noted. A low tear film height of <0.2 mm suggests ADDE.1
On viewing the lashes, note any ingrown, extra and missing lashes. It is useful to check the conjunctiva, which includes the inferior fornix and tarsal, as well as the four quadrants of the bulbar. Staining with fluorescein will help assess the cornea for ulceration, neovascularisation, infiltrates and scarring.19 The presence of cells and flare in the anterior chamber is indicative of intraocular inflammation, which suggests uveitis, a condition that is linked with systemic autoimmune diseases.15 This will need discussion with an ophthalmologist on the day.
An examination to look for clinical associations of other systemic conditions, such as those listed in Figure 1, is useful to determine the treatment pathway.
Investigation
Office tests that can be done include tear break-up time (TBUT) and ocular surface staining.1,22 The Schirmer test can be used to evaluate aqueous tear production.19 If you do not have access to these tests, then referring to a local optometrist is an option.
It is not expected that GPs carry out TBUT tests in the office, as specific equipment is required. The test uses a fluorescein strip dipped in preservative-free normal saline solution.16,22 If there is scope to implement this in your practice, the procedure is performed as follows: The strip is placed in the cul-de-sac with the dye allowed to spread on blinking.19 While the patient is looking straight ahead without blinking, the film is viewed with cobalt blue light and the time between the appearance of a first dry spot or hole and the last blink is confirmed as the TBUT19 (Figure 3C). While there is variability in the times of healthy patients, it is agreed that a cut-off of less than 10 seconds is abnormal and relatively specific in screening for dry eyes.1
Ocular staining can be assessed with a dye such as fluorescein. The staining patterns that could indicate damage are observed over the cornea and conjunctival surface. The criteria of abnormality are >5 corneal spots, >9 conjunctival spots, or lid margin epitheliopathy of ≥2 mm length and ≥25% width.1
The Schirmer test is performed by placing a filter paper over the lower lid margin contacting the ocular surface (Figure 3D). The eyes are closed, and after five minutes, the paper is checked to see the amount of wetting. There is no universal cut-off but tear wetting of <10 mm in five minutes is considered abnormal.9 Its use is variable in practice due to its specificity and sensitivity. Nonetheless, the executive summary from DEWS concluded that this test without anaesthesia is a well-standardised test for providing an estimation of stimulated reflex tear flow.1
Blood tests are usually not required in diagnosing DED; if there is suspicion for underlying systemic illness such as those listed in Figure 3, then specific tests for those conditions will be required as shown.
Treatment
Treatment aims to improve symptoms and effectively improve any related vision disturbance. The aetiology of DED can be multifactorial, which is why treatment should focus on optimising function as well as address any underlying diseases involved. The Royal Australia and New Zealand College of Ophthalmologists has a patient information publication on DED that can be accessed on its website.23
Before considering pharmacological options, it is essential to modify any external factors that contribute to DED. Give the following advice to patients:1,6
- learn about the natural history, chronic nature and outcome expectation (ie symptom relief rather than cure)
- reduce computer use (or lower computer screen height, which reduces lid aperture)
- increase frequency of eye rest
- avoid allergens and irritants – especially any eye drops that have preservatives
- humidify home and work
- minimise air conditioners/heaters
- avoid rubbing the eyes
- cease smoking and avoid second-hand smoke
- reduce alcohol consumption
- ensure that contact lenses are inserted and used correctly
- wear sunglasses or tinted glasses; wrap-around frames should be used in windy conditions
- increase omega-3 fatty acids.
There is evidence to show that a diet rich in omega-3 fatty acids (from fish and plant oils) can improve symptoms. A meta-analysis concluded that due to the anti-inflammatory effect of omega-3 supplementation on the ocular surface, it could help with DED.24
Many pharmacological treatment options are available. For mild disease, it may be enough to use ocular lubricants such as drops, gels or ointments (Table 1). Generally, the dose is one to two drops, or one application, three to four times a day as required. Some patients may need to apply them more frequently. Gels provide more extended relief than drops but can blur vision (less so with ointments). Ointments can be used at night if dry eye affects sleep or occurs on waking. Preservative-free lubricants are preferred for severe dry eye as preservatives can worsen DED and cause epithelium toxicity.25 Adding a lipid-stabilising agent is useful as this can mimic the composition of the natural tear film, which can improve lipid tear film structure and overall stability.26
| Table 1. Available ocular lubricants for dry eye disease |
| Aqueous tear lubricants – main lubricant (available with and without preservatives) |
- Polyethylene glycol (SU and MU; drops, gel)
- Carmellose sodium (SU and MU; drops, gel)
- Hypromellose (SU and MU; drops, gel)
- Sodium hyaluronate (SU and MU; drops)
- Carbomer 980 (SU and MU; gel)
- Polyvinyl alcohol (SU and MU; drops)
|
| Lipid tear supplements – main lubricant |
- Perfluorohexyloctane (MU; drops)
- Propylene glycol and emulsified mineral oil (SU; drops)
- Phospholipid liposomes (spray)
|
| Ointments (no preservatives) |
- Paraffin liquid and white soft paraffin
|
| SU, single use; MU, multi use |
MGD can be optimised with several non-pharmacological methods such as lid massage several times a day, in the direction of meibomian gland opening, warm compresses applied to the eyelids (with eyes closed) for two to five minutes to soften the crusts, and gentle scrubbing of the lashes with eyelid solutions or wipes.14 There is some evidence to suggest that applying chloramphenicol 1% ointment topically to the eyelid margin twice daily for one to two weeks can improve cases of anterior blepharitis if eyelid hygiene measures do not suffice.14 Additionally, doxycycline 100 mg daily for two to four weeks can be used for posterior blepharitis if eyelid hygiene is inadequately controlling symptoms.14 Recently, manuka honey drops have been tried for blepharitis associated DED, and was found to be a useful adjunct.27 However, it may irritate the surface (especially those with DED), which may affect the patient’s adherence.27
More severe and refractory cases need more aggressive interventions – usually managed by the ophthalmologist (in conjunction with a rheumatologist if there is an underlying autoimmune cause), as they will require further investigation and a trial of treatments, which will need close monitoring as well as compounding (refer to Figure 4 for indications for referral).
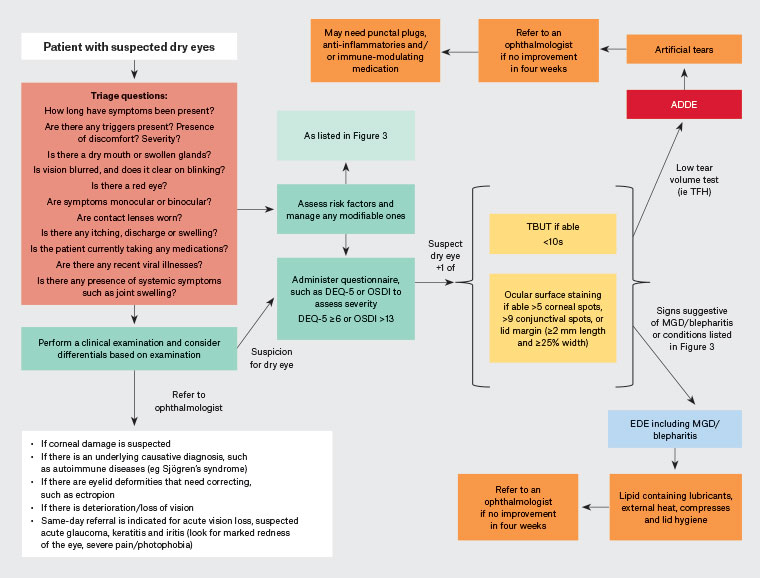
Figure 4. Algorithm approach to dry eye disease. Click here to enlarge
ADDE, aqueous deficient dry eye; DEQ-5, five-item dry-eye questionnaire; EDE, evaporative dry eye; MGD, meibomian gland dysfunction; OSDI, ocular surface disease index; TBUT, tear break-up time; TFH, tear film height
Anti-inflammatories such as topical steroids are occasionally prescribed by an ophthalmologist; if used long term, they increase the risk of glaucoma and early cataracts.12 Ciclosporin is an immune-modulating medication with anti-inflammatory properties that has shown to reduce symptoms and corneal surface damage.28 This, along with punctal plugs, may help severe ADDE.5,9,15 Other treatments include testosterone eye drops and autologous serum eye drops (containing vitamin A growth factors and fibronectin).28 Figure 4 illustrates an algorithm approach to DED.
DED is often underappreciated and underdiagnosed due to the vague nature of the symptoms. It is now known that it has a multifactorial aetiology, which is why symptom questionnaires are useful in primary care settings to diagnose DED and grade its severity accurately. GPs can manage mild DED with good advice on reducing modifiable risk factors, while more severe disease should alert the doctor to refer to an ophthalmologist.
Conclusion
DED is an important condition that needs to be recognised in primary care settings. Treatment options are extensive; if commenced early, they can improve the patient’s quality of life. The diagnosis needs to be explored in all autoimmune diseases, including Sjögren’s syndrome and non-Sjögren’s syndrome. Incorporating this in general practice management care plans may provide opportunities for the GP to discuss this debilitating condition.
Key points
- DED has a multifactorial aetiology.
- It is important to identify risk factors for the development of DED.
- DED can be broadly divided into ADDE and EDE.
- DED questionnaires are important tools to use as an adjunct to routine history, examination and investigations.
- Treatment involves tackling modifiable risk factors with the addition of appropriate ocular lubricants and managing any underlying cause.
- Referral to an ophthalmologist is warranted for people with refractory and severe symptoms.