Background
Parkinson’s disease is characterised by a complex array of motor and non-motor symptoms. Motor symptoms are often prioritised for assessment and treatment. Growing evidence highlights the importance of recognising the impact of non-motor symptoms on the person’s quality of life.
Objective
The aim of this article is to increase awareness of the importance of identifying and addressing non-motor symptoms experienced by people living with Parkinson’s disease.
Discussion
A vignette developed in collaboration with a person living with Parkinson’s disease and his wife provides an ‘insider perspective’. Regular assessment and monitoring of non-motor symptoms enable the clinician to support the implementation of effective interventions. Team-based care and connections with Parkinson’s support groups are essential to enable the person to live well with Parkinson’s disease and provide informal carers with the support and information needed.
Parkinson’s disease is a complex neurodegenerative disorder that affects people in very diverse ways and is the second most common neurological condition worldwide.1 The management of Parkinson’s disease for many years has focused largely on treating and improving motor symptoms – bradykinesia combined with either rigidity, resting tremor or both.2 Non-motor symptoms – including anxiety and depression – typically pre-date the emergence of motor signs by years.3,4 Usually, a person presents with a combination of non-motor and motor symptoms4,5 that affect the person’s level of disability and health-related quality of life (QoL).3,6,7 A diagnosis of Parkinson’s disease should be inextricably linked to therapeutic interventions that focus on improving a patient’s QoL and address both motor and non-motor symptoms. While it is essential to understand and adequately treat motor symptoms, this article draws attention to the importance of identifying and addressing non-motor symptoms experienced by the person living with Parkinson’s disease. A vignette developed in collaboration with a person living with Parkinson’s disease and his wife provides an ‘insider perspective’ (Box 1).
| Box 1. Vignette as told by Tony and Jenny |
For 28 years, Tony, aged 62 years, and Jenny ran a successful carpet cleaning business. Outside work Tony was active, playing A grade tennis twice weekly and swimming regularly. In 2014, Tony experienced ‘terrible anxiety’; the couple attributed this to stress, deciding to sell the business. Tony developed depression. In 2016, he commenced antidepressants, trying three different types with no benefit. The company sold, and contrary to the agreement reached with the new owners, the workload did not ease. Tony’s mental health continued to deteriorate.
Jenny noticed Tony’s changing gait. Tony attributed his loss of smell to chemicals used for carpet cleaning; his depression deepened. He said, ‘I can’t live like this’. Insomnia worsening, he took to occasionally walking the streets at 1 o’clock and 2 o’clock each morning. Antidepressants appeared to make his insomnia worse. Counselling made no difference. The general practitioner referred Tony to a neurologist. Jenny suspected Parkinson’s disease. The struggle with anxiety and depression delayed him seeing a neurologist until 2017.
A diagnosis of Parkinson’s shocked Tony, who thought it was stress and overwork. The neurologist identified slowing gait and ‘cogwheel rigidity’, initially prescribing three different types of medication without any improvement to Tony’s motor symptoms. Two of these were dopamine agonists: 1) a rotigotine patch was initially commenced at 2 mg, titrating slowly up to 8 mg over six months, and caused a skin rash; 2) pramipexole was associated with somnolence and sudden onset of sleep; and 3) rasagiline, a monoamine oxidase type-B (MAO-B) inhibitor, caused excessive daytime sleepiness and nausea. Tony’s mobility further decreased; he was unable to dress, clean his teeth or brush his hair without Jenny’s help. His mood deteriorated further. Soon after an admission to hospital in 2018 with constipation and urinary retention, the Parkinson’s nurse reassessed Tony, then consulted his neurologist, advising that Tony’s struggle with immobility, depression and anxiety was affecting his quality of life. The neurologist reassessed Tony, commencing levodopa and referring Tony to a psychiatrist who was unable to help. Within three months, Tony was able to move freely, his anxiety and depression much improved.
Tony now sees a movement disorder neurologist. Tony and Jenny also contacted Dr Constantini, an Italian neurologist (died of COVID-19, May 2020) who recommended high-dose thiamine hydrochloride, providing rapid relief of Tony’s constipation. With ongoing support from the Parkinson’s nurse, Tony is a ‘lot more hopeful’. Regular Parkinson’s medications and antidepressants provide pharmacological support, and Tony’s mental health is ‘heaps better now’. Jenny and Tony are active in Parkinson’s support groups, locally and online. Tony is enrolled in three boxing and two PD Warrior sessions weekly, plays tennis and walks regularly. Tony says, ‘I feel the need to exercise to maintain my physical and mental wellbeing; it helps with my quality of life. I get exhausted pushing my body, but then I can sleep well at night.’ |
The aim of this article is to deepen knowledge of:
- the prevalence of non-motor symptoms in Parkinson’s disease
- the impact of non-motor symptoms on the person’s QoL
- validated approaches to the assessment of non-motor symptoms
- non-pharmacotherapeutic interventions for non-motor symptoms
- a team approach to care.
Early and timely referral to a neurologist or movement disorder specialist is imperative to confirm the diagnosis and establish a shared management plan for the person with Parkinson’s disease.8 A confirmed diagnosis of Parkinson’s disease is needed to link together non-motor symptoms that the person may have also been experiencing for years before diagnosis. A diagnosis also allows the patient and family to explore interventions to address non-motor symptoms that can be somewhat puzzling without the overarching diagnosis. The phases of Parkinson’s disease are Diagnosis, Maintenance, Complex (autonomic problems prominent, five or more doses of medication, or infusion therapy) and Palliative.9 Identifying the phase of Parkinson’s disease enables further tailoring of the appropriate supportive interventions.
Assessing and treating both motor and non-motor symptoms are vital to the effective management of Parkinson’s disease. General practitioners (GPs) are well placed to coordinate a holistic approach to care for those with Parkinson’s disease. The focus of GP management has been described as follows: 8
- Encourage a healthy lifestyle.
- Help and guide the patient, caregiver and family to understand the disease.
- Manage the symptoms of Parkinson’s disease throughout the disease.
Medication prescription for Parkinson’s disease is dependent on a person’s level of disability from the motor symptoms, and the impact Parkinson’s disease is having on their QoL. The choice of medication should consider a person’s age, lifestyle, occupation and medical history.8,10 Dopamine replacement therapy is the most effective treatment for Parkinson’s disease. When motor symptoms are affecting a person’s QoL, first-line treatment in the early stages of Parkinson’s disease is levodopa. When motor symptoms are not affecting QoL, consideration can be given to dopamine agonists, levodopa or monoamine oxidase B (MAO-B) inhibitors; these can also be included as potential adjuncts later in the progression of Parkinson’s disease.10,11
Motor symptoms have been described as the ‘tip of the iceberg’.12 Focusing solely on motor symptoms severely disadvantages the patient, missing the opportunity to address non-motor symptoms (Box 2) affecting the person’s QoL and potentially contributing to further functional decline. In 2020, Still et al described the relationship between severity of non-motor symptoms and functional disability as reported by people living with Parkinson’s disease.13 The researchers highlighted the possibility that clinician measures and perceptions may not reflect the person’s experience of living with Parkinson’s disease and the impact of non-motor symptoms.
Table 1 presents a sample of the extensive research reporting the pervasiveness of non-motor symptoms experienced by people living with Parkinson’s disease and correlation with QoL.
| Box 2. Non-motor symptoms of Parkinson’s disease44 |
- Cognitive impairment
- Cardiovascular dysfunctions
- Urinary problems
- Hallucinations and delusions
- Sexual dysfunction
- Anxiety and depression*
- Gastrointestinal symptoms (constipation*)
- Sleep disturbance (rapid eye movement sleep behaviour disorder*)
|
Note: Non-motor symptoms can be more troublesome and disabling than motor symptoms.
*These symptoms can occur years before a diagnosis of Parkinson’s disease. |
| Table 1. Prevalence of non-motor symptoms and correlation with QoL |
| Study |
Sample size |
Findings |
| Chaudhuri et al (2021)44 |
n = 75 |
100% of patients with Parkinson’s disease identified at least one non-motor symptom
Higher prevalence of non-motor symptoms in those with disease duration >9 years |
| Gulunay et al (2020)12 |
n = 75 |
100% of patients with Parkinson’s disease identified at least one non-motor symptom:
- fatigue (58%)
- anxiety (56%)
- leg pain (38%)
- insomnia (37%)
- urgency/nocturia (35%)
- drooling (31%)
|
| Müller et al (2013)17 |
n = 166 |
Non-motor symptoms remain a concern three years after diagnosis
Affect quality of life (QoL) as measured by the 36-Item Short Form Health Survey
Non-motor symptoms should be treated as they contribute primarily to reduced QoL |
| Nicoletti et al (2017)7 |
n = 272 |
Decreased QoL score correlated with Parkinson’s Fatigue Scale, Beck Depression Inventory, Unified Parkinson’s Disease Rating Scale Section I, Parkinson’s Disease Sleep Scale |
Emerging research is identifying different phenotypes of Parkinson’s disease. Awareness of these phenotypes enables clinicians to be mindful of the non-motor and fall-related features linked to specific phenotypes, such as those associated with postural instability–gait difficulty (PIGD) and tremor-dominant subtypes.14 This area of research is in the early stages; however, findings suggest PIGD is significantly linked to fatigue, gastrointestinal dysfunction and increased fall-related risks. While it is essential to consider the phenotype of the disease, it is also important to assess all people living with Parkinson’s disease for non-motor symptoms. For example, Abou Kassm et al examined anxiety as it relates to Parkinson’s disease and suggest that one-third of patients with Parkinson’s disease meet the criteria for an anxiety disorder.15 This may present before a diagnosis of Parkinson’s disease is made. Anxiety is also a predictor of low QoL and is often overlooked or confused with depression.
The Non-Motor Symptoms Scale (NMSS) is a comprehensive assessment capturing many of the non-motor symptoms associated with Parkinson’s disease. More than 100 clinical trials and studies have shown strong correlations between NMSS burden and health-related QoL measures. The NMSS covers 30 non-motor symptoms grouped into nine domains, providing a roadmap to assist quantifying the far-reaching burden of non-motor symptoms in people with Parkinson’s disease.1–3 The shortened version of the scale can be accessed online from Parkinson’s UK (www.parkinsons.org.uk/sites/default/files/2021-04/Fillable%20NMS%20questionnaire.pdf). Using a screening tool regularly helps identify non-motor symptoms, which then allows clinicians to tailor an individualised plan to address the specific needs of the person.2,4 A 2019 medicine review provides a comprehensive update of evidence-based pharmacological recommendations for an extensive range of non-motor symptoms experienced by people with Parkinson’s disease.16 Referral to this resource is recommended; it can be accessed online (https://movementdisorders.onlinelibrary.wiley.com/doi/epdf/10.1002/mds.27602).
Recent findings suggest that non-pharmacological interventions to address non-motor symptoms may be underrecognised and underemphasised by both doctors and patients.7,17,18 However, a growing body of research shows the benefits of a range of different interventions. Exercise in many forms has emerged as a prominent adjunct therapy, helping alleviate symptoms associated with Parkinson’s disease. These include the motor and non-motor symptoms; reducing falls risk; improving gait and strength, cognition and sleep quality; reducing stress and anxiety; and improving disease progression and QoL.19 The exercise types include aerobic exercise, dancing, aquatic, yoga and boxing,19–32 and Kalyani et al33 propose benefits may arise because of the release of dopamine and sometimes the ongoing engagement with other people.
Speech problems affect up to 51–90% of people with Parkinson’s disease. Dysarthria is common, characterised by reduced loudness, limited respiratory support, monotone voice and reduced voice quality. Speech pathology intervention programs and singing and voice therapy have the potential to improve voice-related QoL and respiratory function, as well as increase voice loudness and communication.22,34,35
Another important issue requiring careful attention relates to the need for caregiver and social support. Insufficient psychosocial support may be associated with poorer psychosocial outcomes for people with Parkinson’s disease.36 However, intimate relationships with loved ones with Parkinson’s disease are increasingly characterised by reduced mutuality. The individual becomes an informal carer rather than a partner and experiences high levels of burden and strain.37–40 Given the progressive nature of Parkinson’s disease and the increasing burden, especially as the patient moves from the maintenance to complex phase, regular assessment of the caregiver’s needs in conjunction with referral to additional support services is imperative.
Early referral to a specialist Parkinson’s nurse whose expanded role of advanced practice can support self-management and an enhanced QoL from diagnosis and through the complexities that arise as the disease progresses is recommended. The specialist nurse can likewise support the person and their family when transition to palliative care is needed. Engagement with a Parkinson’s support group can offer additional information and resources and help to reduce social isolation through friendship and shared experiences. GPs can encourage both the person with Parkinson’s disease and family members to contact the peak body for Parkinson’s in the state as a further source of information, and for links to relevant support services.
The complexity of Parkinson’s disease requires individualised care. Figure 1 presents the key components of effective team-based care.41–43
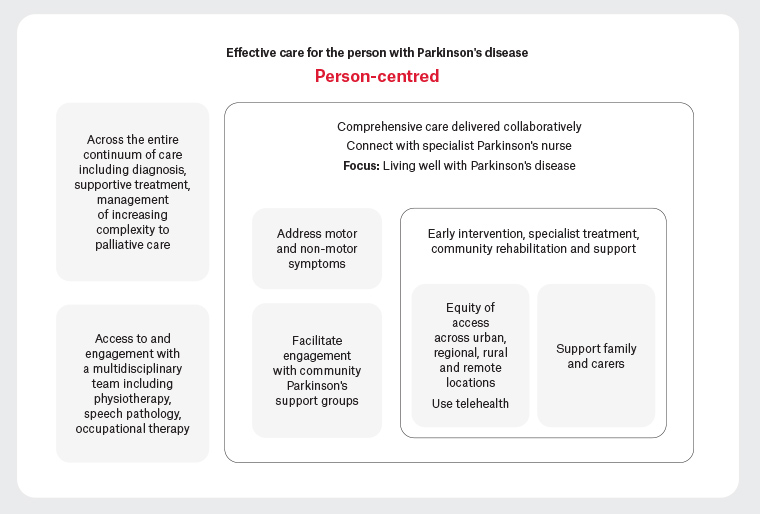
Figure 1. Effective team-based care for a person living with Parkinson’s disease39,40
Conclusion
The complexity and progressive neurodegeneration that characterise Parkinson’s disease have a severe impact on the QoL of the person living with the disease. Family members and informal carers experience increasing levels of caregiver burden as the person’s physical, cognitive and psychological health deteriorates. Careful attention across each phase of Parkinson’s disease, with regular assessment of non-motor symptoms and a team-based approach to individualised care, is key to maximising the person’s QoL and reducing emergency presentations to hospital. It is important to ensure that the team includes a specialist Parkinson’s nurse and allied health professionals. Wherever possible, the GP should ensure referral to a movement disorder neurologist or geriatrician and request regular review of medication regimens. It is critical to listen carefully to the person’s experience and engage regularly with the person’s informal caregiver and family to ensure adequate supports are in place. Linking the person and their carer to the Parkinson’s peak body within their state or region and the local support group will help to reduce social isolation and enable access to additional consumer-focused information.
Key points
- The non-motor symptoms of Parkinson’s disease are more disabling than the motor symptoms.
- Dopamine replacement therapy remains the most effective pharmacological intervention in Parkinson’s disease.
- Focused exercise programs have been shown to alleviate Parkinson’s symptoms.
- GPs can enable a connection with specialist Parkinson’s nurses and Parkinson’s support groups.
- It is important to ensure adequate support services are in place for informal carers and family