Case
A woman aged 79 years presented with a four-day history of a new gradual onset bilateral temporal headache of moderate severity. The pain was ‘sharp and shooting’ in nature, lasting between a few seconds and a couple of minutes, with radiation to her cheeks and neck. Her pain came in intermittent episodes every 3–5 minutes. She did not have any visual changes or any other neurological symptoms. She was otherwise well, with no relevant medical history, and did not have any infective symptoms. The patient did not take any regular medications, and she had no allergies. Physical examination was unremarkable, and her vital signs were within normal limits. There was no temporal tenderness, neck stiffness, disc oedema on fundoscopy or focal neurological deficit. Best corrected visual acuity was 6/9 in both eyes with equal and reactive pupils bilaterally.
Question 1
What is your differential diagnosis?
Question 2
What further investigations would you undertake?
Answer 1
An effective acronym of features that identify secondary headaches of significant morbidity and mortality in all ages is SNOOP4 (Table 1). Relevant secondary headache differentials that must be excluded include giant cell arteritis (GCA), cerebral ischaemia, infection (meningitis, encephalitis), intracranial haemorrhage or neoplasm cardiac cephalalgia and acute glaucoma. The International Classification of Headache Disorders (ICHD-3, Table 2) gives a broad framework of differentials for other secondary headaches. The age of this patient is a red flag, as serious secondary headaches are more likely in the elderly1 and must be excluded. Further investigations must be undertaken. Primary headaches are still epidemiologically more common in older adults but should only be considered after excluding secondary causes.1 Tension-type headaches are most common, followed by migraine headaches.
| Table 1. Red flag features in headaches (SNOOP4) |
| Mnemonic |
Presentation |
| Systemic symptoms |
- Unexplained fever, chills, weight loss, myalgia
- Previous malignancy, immunosuppression
|
| Neurological symptoms |
- Complaints of motor weakness, sensory loss, diplopia or ataxia
- Abnormal neurological examination
|
| Onset sudden |
- Thunderclap headache, sudden in onset, with peak intensity in <1 minute
|
| Onset after age 50 years |
- New onset after the age of 50 years
|
| P 4 |
- Progressive headache or substantial change
- Precipitated by Valsalva manoeuvre
- Postural aggravation
- Papilloedema
|
| Table 2. Primary and secondary headaches7 |
| Primary headaches |
Secondary headaches (some examples) |
| Migraine headache |
Cranial or cervical vascular disorder (giant cell arteritis, cerebral venous thrombosis, cerebral ischaemia, intracranial haemorrhage) |
| Tension-type headache |
Non-vascular intracranial disorders (intracranial neoplasia) |
| Trigeminal autonomic cephalalgias |
Infections (meningitis, encephalitis) |
| Other primary headache disorders (primary stabbing headache, nummular headache) |
Trauma (injury to the head and neck, whiplash) |
| Substance use or withdrawal (medication overuse headache) |
| Disorders of homoeostasis (fasting, hypothyroidism, hypertension, cardiac cephalalgia) |
| Disorders of the cranium, neck, eyes, ears, nose, sinuses, teeth, mouth or other facial or cervical structures (angle closure glaucoma, rhinosinusitis, temporomandibular joint dysfunction) |
| Painful lesion of the cranial nerves (trigeminal neuralgia) |
Answer 2
The initial workup should include baseline bloodwork including a full blood examination to evaluate the platelet count in addition to biochemical inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). The ESR should be age adjusted (ie the upper limit is calculated by [age in years + 10] ÷ 2). Given the onset of a new headache in an elderly patient, radiological evaluation in the form of a non-contrast computed tomography scan of the brain is appropriate to rule out an intracranial process (haemorrhage or neoplasm).
Case continued
Initial blood tests demonstrated a white cell count (WCC) of 11.04 × 109/L, platelet count of 186 × 109/L, ESR of 33 mm/hr and CRP of 37 mg/L. The ESR was within normal limits when the upper limit was adjusted for age using the aforementioned formula. The CRP was mildly elevated but deemed to be non-specific (reference range <3 mg/L). Her non-contrast brain CT scan was appropriate for her age, with no evidence of acute intracranial pathology. She was given a provisional diagnosis of trigeminal neuralgia, provided pain relief and discharged to be followed up in the community.
Nine days later she presented with ongoing, progressively worsening headaches. The headaches were now associated with jaw claudication, scalp hypersensitivity and temporal tenderness. Laboratory values demonstrated an ESR of 66 mm/hr, beyond the upper limit of normal for her age and sex, and an elevated CRP of 125 mg/L. She was started on a prednisolone dose of 50 mg/day by the local rheumatologist. The patient was then referred for temporal artery ultrasonography.
Question 3
What further investigations would you undertake now?
Answer 3
The primary differential to be considered in this patient is GCA, which has a strong association with polymyalgia rheumatica (PMR).1 Laboratory investigations including ESR, CRP, haemoglobin and platelet count must be performed in all patients with suspected GCA.1 In 5–11% of patients who are diagnosed with GCA, ESR and CRP are within normal limits.2
Histopathological evidence from temporal artery biopsy (TAB) is the gold standard of GCA diagnosis. However, TAB is limited by the presence of skip lesions. From recent evidence, the optimal diagnostic length is 2 cm.3 A recent systematic review and meta-analysis showed a sensitivity of 77% for TAB.4 Given the protean nature of GCA and relatively low sensitivities of investigations and histopathology, diagnosis requires a combined approach using clinical, laboratory, imaging and histopathological data.
Colour Doppler ultrasonography (CDUS) captures 0.1 mm resolution images of the cranial arteries (temporal, facial, occipital and vertebral). Mural oedema (the ‘halo sign’), which is identified on CDUS, can confirm the diagnosis of GCA. Although it is being increasingly adopted in Europe, CDUS is limited by operator dependence and clinical experience. Consequently, CDUS should be used in tertiary settings in patients with very high or very low clinical suspicion of GCA and should not replace TAB. Patients with a negative CDUS result with high clinical suspicion of GCA should still undergo TAB.
The utility of CT of the brain in GCA is low. Other imaging modalities, including CT angiography and positron emission tomography, have greater utility. Exploration of these modalities, however, is outside the scope of this article.
Case continued
Temporal artery CDUS (Figure 1) did not demonstrate the ‘halo sign’. The patient underwent a TAB with the local vascular surgeon. This demonstrated features of GCA, with a cuff of chronic inflammatory cells and histocytes within the adventitia, encroaching through the media into the internal elastic lamina and intima (Figure 2).
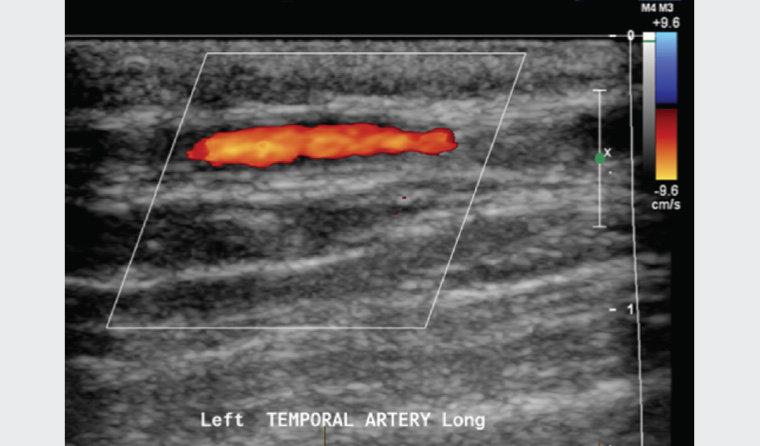
Figure 1. Normal ultrasound of the temporal arteries
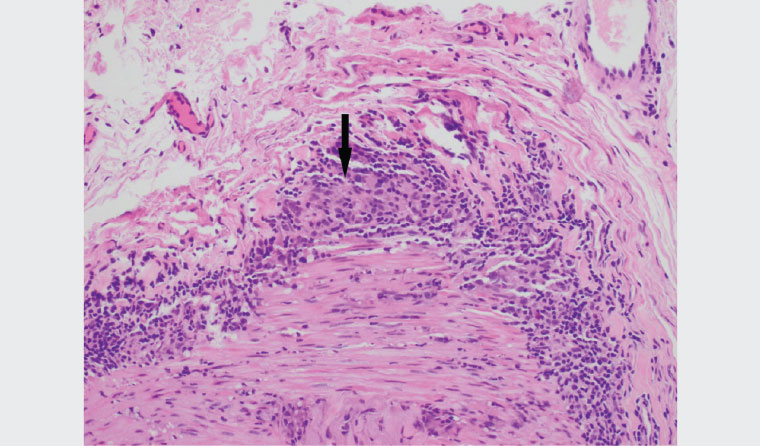
Figure 2. High-power cross-section of temporal artery biopsy illustrating areas of granuloma formation (arrow)
Question 4
How would you manage the patient’s current presentation?
Question 5
What are the potential complications of long-term prednisolone therapy?
Answer 4
Rapid initial treatment is crucial to reduce the risk of sight-threatening complications in GCA. Patients without vision loss at diagnosis should be treated with oral prednisolone at 1 mg/kg to a maximum of 60 mg/day. For patients with threatened or established visual loss at diagnosis, intravenous methylprednisolone should be commenced at doses of 500 mg to 1000 mg each day for three days. The management of GCA should be undertaken in collaboration with local inpatient or specialist services.
Answer 5
The initial prednisolone dose is maintained for a period of 2–4 weeks and gradually reduced to prevent relapse in symptoms. The average tapering regimen takes 2–3 years, with average cumulative doses of 5000–6000 mg per patient.5 Up to 90% of patients who undergo a course of tapering prednisolone will experience at least one side effect.6 Glucocorticoid complications can include the development of osteoporosis, diabetes, dyslipidaemia, gastrointestinal events, psychiatric and cognitive disturbances and immunosuppression.7 There is a need to monitor these complications. A suggested schedule for monitoring is provided in Table 3. Steroid-sparing therapies exist but require specialist input. Tocilizumab is a monoclonal antibody that has shown reduced steroid burden and has been listed on the Pharmaceutical Benefits Scheme.
| Table 3. Monitoring of patients taking long-term corticosteroids8 |
| Baseline monitoring |
Weight
Height
Body mass index
Blood pressure |
Complete blood count
Glucose
Lipids
Bone mineral density (BMD) |
| Subsequent monitoring |
Bone health |
Annual height assessment for fragility fractures
BMD four months post–steroid initiation
- If stable: to assess every 2–3 years
- If decreased: assess annually
Fracture Risk Assessment Tool (FRAX) to estimate fracture risk |
| Dyslipidaemia and cardiovascular risks |
Assess lipids one month after steroid initiation
Reassess every 6–12 months
Australian absolute cardiovascular risk calculator to assess five-year cardiovascular risk |
| Hyperglycaemia/diabetes |
Screen opportunistically for symptoms of diabetes
Monitor glucose parameters 48 hours after steroid initiation
- Then 3–6 months for the first year, annually thereafter
|
| Ophthalmological examination |
Referral for annual eye examination
Consider risk factors for glaucoma (family history, high myopia) and refer early if at high risk |
| Falls risk |
Assess people aged >65 years every 12 months. If previous falls or high risk of falls then every six months. |
Key points
- GCA should be considered in all patients over the age of 50 years with the symptoms of headaches, visual disturbances, jaw claudication, unexplained fever and other constitutional symptoms and signs.
- CDUS has become an increasingly used modality for first-line investigation in Europe. In Australia, this modality should be undertaken in specialist centres with extensive expertise, in collaboration with local specialist services. CDUS should not replace TAB.
- GCA is a chronic disease requiring multidisciplinary input. The monitoring and management of secondary complications is necessary.