Discussion
All patients with evidence of metabolic dysregulation are at risk of MAFLD. Diagnosis requires fulfillment of the new diagnostic criteria for MAFLD. Most patients with MAFLD die as a result of cardiovascular disease or extrahepatic cancer, but liver-related outcomes including cancer can develop, especially in those with more advanced stages of fibrosis. There is no approved medication therapy for MAFLD, and so management focuses on lifestyle intervention, diabetes control, treatment to target of risk factors such as dyslipidaemia, and avoidance of smoking and alcohol. Most patients with MAFLD are best managed in primary care.
In 2020, Australian researchers led the development of an international consensus to rename and more precisely and positively define fatty liver disease associated with metabolic dysregulation.1 What was previously termed non-alcoholic fatty liver disease (NAFLD) is now referred to as metabolic (dysfunction) associated fatty liver disease (MAFLD).1,2 The consensus panel also proposed a set of simple positive criteria for disease diagnosis (Figure 1). Rather than being a disease that is identified when all other causes of liver disease are excluded, if a person meets the diagnostic criteria, they have MAFLD.1,3 Importantly, MAFLD allows a patient to have dual (or more) aetiologies for their liver disease (eg alcohol and MAFLD, or hepatitis C and MAFLD), something that by definition is not possible with the old NAFLD term.3 Previous definitions of NAFLD in international guidelines required the presence of steatosis in >5% of hepatocytes in the absence of significant ongoing or recent alcohol consumption and other known causes of liver disease.4
MAFLD is a consequence of increased fat deposition in the liver that is caused by metabolic dysregulation.1 If not adequately treated, approximately one in 5–10 people will develop liver fibrosis over time, which can progress to cirrhosis, liver failure or liver cancer.4 Importantly, as a result of the link with metabolic dysregulation, people with fatty liver are at an increased risk of extrahepatic complications including cardiovascular disease, type 2 diabetes and cancers (Box 1).5 The clinical hepatic and extrahepatic manifestations of MAFLD are influenced by a multitude of disease modifiers including age, sex, ethnicity, diet, microbiota, genetics and metabolic health.6 A major leap in recent understanding has been that up to 40% of individuals with MAFLD have a normal weight by body mass index (BMI) criteria.4
| Box 1. Risk factors for metabolic (dysfunction) associated fatty liver disease3 |
Major risk factors
- Central adiposity, overweight/obesity, insulin resistance, type 2 diabetes, atherogenic dyslipidaemia, arterial hypertension, metabolic syndrome
- Dietary factors (high-calorie diets rich in saturated fats and cholesterol, soft drinks high in fructose, highly processed foods)
- Sedentary lifestyle/occupation/low level of physical activity
- Sarcopenia
Disease associations
- Cardiovascular disease
- Cerebrovascular disease
- Chronic kidney disease
- Osteoporosis
- Cancer
- Cognitive changes
- Hyperuricemia
- Hypothyroidism
- Polycystic ovarian syndrome
- Hypopituitarism
- Sleep apnoea syndrome
- Polycythaemia
- Gut dysbiosis
|
Since MAFLD is common, it frequently can and does coexist with other liver disease including alcoholic liver disease (>60% of Australians consume more than minimal amounts of alcohol) or viral hepatitis.7–9 Liver disease from dual aetiologies has an accelerated natural history when compared with MAFLD alone, and therefore an increased risk of adverse outcomes.7 An example is recent mounting evidence that even low ‘safe limit’ alcohol intake is associated with an increased risk for cirrhosis and cancer in patients with MAFLD.9,10 All patients diagnosed with MAFLD should be screened for other potential contributors to chronic liver disease.
Diagnosis
All patients with metabolic abnormalities are at risk of MAFLD. Patients should be screened for MAFLD if they have type 2 diabetes, are overweight or obese, or have metabolic risk abnormalities, even if they are lean (Figure 1).3–5
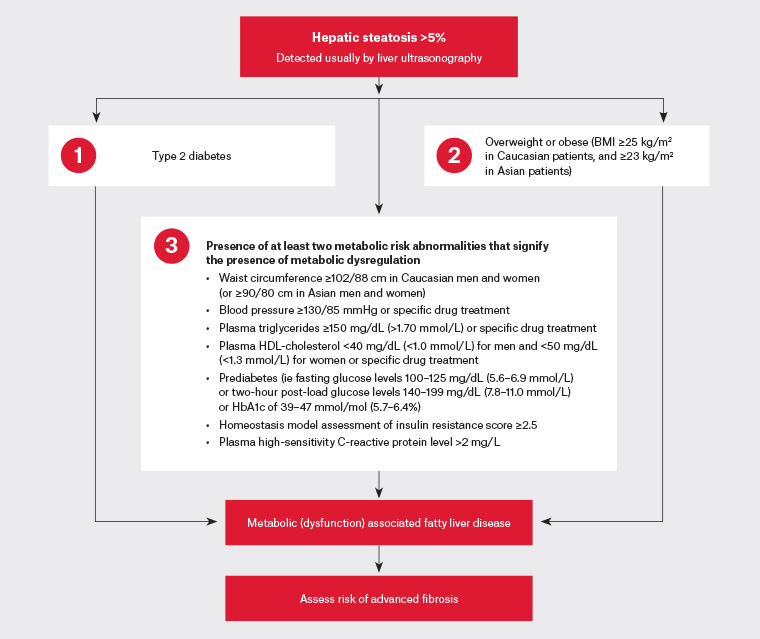
Figure 1. Three separate diagnostic pathways for metabolic (dysfunction) associated fatty liver disease in patients with hepatic steatosis. Click here to enlarge
BMI, body mass index; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein
The most widely used detection modality for the presence of steatosis is ultrasonography, which is recommended as the first-line tool for diagnosis.11 Ultrasonography is usually sufficient to diagnose the presence of liver fat; however, the sensitivity of ultrasonography is limited when steatosis is <20% of hepatocytes, and performance is suboptimal in individuals with a BMI of >40 kg/m2.12 If available, liver stiffness should be measured by elastography as it is a more sensitive tool.11 Currently there are no robust liquid biomarkers that can confidently and easily be used outside of research settings to diagnose the presence of hepatic steatosis. Liver biopsy remains the gold standard test for both diagnosis and staging of the disease but is infrequently used, even in specialist hepatology practice.11 It can, however, be useful to rule out other liver diseases, especially when the clinical picture is atypical for MAFLD, such as high aminotransferases with little metabolic burden.
Once a diagnosis of MAFLD is made, the next step is to assess the stage of fibrosis, as this is the most important predictor of liver-related morbidity and mortality in patients, with the highest risk among those with cirrhosis.13 Fibrosis staging can be performed by using a combination of non-invasive liver scores (eg Fibrosis-4 [FIB-4]14 and NAFLD Fibrosis Score [NFS],15 refer to Table 1) or liver stiffness measurement by elastography (Table 2).16,17 The FIB-4 and NFS can be easily calculated using smartphone apps. Although non-invasive liver scores have only modest accuracy, they are inexpensive, comprise clinical and routine laboratory parameters, and have good negative predictive values to exclude advanced fibrosis.18,19 The availability of liver elastography may be limited for general practitioners (GPs) in the community; however, many imaging practices are able to add elastography to an abdominal ultrasonography request if it is specified and their machine has the capability.
| Table 1. Fibrosis score comparisons |
| |
NAFLD Fibrosis Score (NFS)15 |
Fibrosis-4 (FIB-4) score14 |
| Parameters |
|
|
| Age |
Y |
Y |
| AST |
Y |
Y |
| ALT |
Y |
Y |
| Platelet count |
Y |
Y |
| BMI |
Y |
N |
| Albumin |
Y |
N |
| Impaired fasting glucose/diabetes |
Y |
N |
| Calculation |
–1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio – 0.013 × platelet count (× 109/L) – 0.66 × albumin (g/dL) |
Age (years) × AST (U/L) ÷ platelets (109/L) × √ALT (U/L) |
| Results |
|
|
| Rules out significant fibrosis |
<–1.455 |
<1.3 |
| Predicts significant fibrosis |
>0.676 |
>3.25 |
| Positive predictive value for significant fibrosis |
82–90% |
75% |
| Negative predictive value for significant fibrosis |
88–93% |
95% |
| ALT, alanine transaminase; AST, aspartate aminostransferase; BMI, body mass index; N, no; NAFLD, non‑alcoholic fatty liver disease; Y, yes |
| Table 2. Common liver elastography measurements in metabolic (dysfunction) associated fatty liver disease16,17 |
| Supersonic shear imaging (SSI) elastography or two-dimensional shear wave elastography (SWE) |
Cut-off |
Sensitivity, specificity |
Reliable result |
| ≥F2 |
6.3 kPa |
90%, 50% |
79.7% |
| 8.7 kPa |
71%, 90% |
| ≥F3 |
8.3 kPa |
91%, 71% |
| 10.7 kPa |
71%, 90% |
| F4 |
10.5 kPa |
90%, 72% |
| 14.4 kPa |
58%, 90% |
| Vibration controlled transient elastography (VCTE) or transient elastography (TE), marketed as Fibroscan |
|
|
|
| ≥F2 |
6.2 kPa |
90%, 45% |
81% |
| 9.8 kPa |
60%, 90% |
| ≥F3 |
8.2 kPa |
90%, 61% |
| 12.5 kPa |
57%, 90% |
| F4 |
9.5 kPa |
92%, 62% |
| 16.1 kPa |
65%, 90% |
| Acoustic radiation force impulse (ARFI) elastography or point wave shear wave elastography (pSWE) |
|
|
|
| ≥F2 |
0.95 m/s |
90%, 36% |
76.6% |
| 1.32 m/s |
56%, 91% |
| ≥F3 |
1.15 m/s |
90%, 63% |
| 1.53 m/s |
59%, 90%, |
| F4 |
1.3 m/s |
90%, 67% |
| 2.04 m/s |
44%, 90% |
General management
Most patients with MAFLD do not require specialist hepatology referral and are best managed holistically in primary care. There is no approved pharmacotherapy for MAFLD. Hence, current management involves reducing the burden of metabolic dysregulation to reduce both liver injury and adverse extrahepatic outcomes.20 The cornerstone of current therapy remains lifestyle modification including dietary change, weight loss and structured exercise intervention.21
Lifestyle intervention programs focusing on weight loss can reduce liver fat content, with subsequent resolution of steatohepatitis and fibrosis, and improve a patient’s quality of life in a dose-dependent manner. A study of 293 patients showed an improvement in liver fibrosis in approximately 90% of those achieving weight loss of >10%.22 Additionally, in 45% of patients who achieved weight loss of >10%, there was an improvement in fibrosis.22 The overall aim of lifestyle intervention should be a hypocaloric diet (500–1000 kcal deficit) resulting in weight loss (up to 1 kg/week).21 There is no strong evidence to support a particular dietary approach for the resolution of MAFLD.19
In terms of exercise for the resolution of MAFLD, there is no clear consensus on the optimal type, intensity or volume. There appears to be a dose-dependent relationship between exercise volume and reduction in hepatic fat, while exercise has clear benefits for cardiovascular health, which is critical as cardiovascular disease is the leading cause of death for people with MAFLD.23 In a study performed by Oh et al in 2015, individuals who exercised more than 250 minutes/week had a greater reduction in hepatic steatosis when compared with individuals who exercised for 150 minutes/week.23
An important strategy is to reduce or remove medications that are steatogenic with long-term use, such as corticosteroids, valproic acid, methotrexate and amiodarone.11 These decisions should be made after consideration of each patient’s risk versus benefit profile for their underlying condition, and in discussion with the relevant non-GP specialists.
MAFLD is one of many disease entities associated with metabolic dysregulation; others include cardiovascular and renovascular disease. As part of a diagnosis of MAFLD, patients require evaluation for cardiovascular risk, with particular attention to dyslipidaemia, hypertension and diabetes.11 Management of these associated entities, and referral to relevant non-GP speciality care if required, is part of the essential treatment plan for patients with MAFLD. Currently there are no head-to-head evidence-based treatments for dyslipidaemia, hypertension and type 2 diabetes in the setting of MAFLD.24,25
Disease-specific management
There are no evidence-based medications for the treatment of MAFLD. However, MAFLD is a ‘hot’ area for clinical trials, with >200 ongoing trials in various phases worldwide.26 Several of the compounds being investigated have shown promising results in early phase trials, but phase III trial data are awaited. Highly promising are the newer insulin-sensitising agents, such as GLP-1 receptor agonists.22 Metformin does not improve liver histology.25
Bariatric and metabolic therapies (both endoscopic and surgery) can be beneficial for patients with MAFLD, reducing steatosis, inflammation and fibrosis. According to recent systematic reviews and meta-analyses, resolution of hepatic steatosis is seen in >75% of patients following bariatric and metabolic therapies.27,28 The lack of randomised controlled trials to investigate bariatric surgery and other procedures for the treatment of MAFLD currently limits the risk versus benefit evaluation; therefore, bariatric surgery should not be used with MAFLD as the primary indication.
Long-term monitoring
There is no current accepted consensus for the optimal strategy for monitoring patients with MAFLD. Fibrosis progression in MAFLD is slow, progressing at a rate of around 0.12 stages per year (stage 0 being a normal liver, stage 3 being pre-cirrhosis and stage 4 being cirrhosis).11 Patients with stage 2–4 fibrosis should have the benefit of a non-GP specialist opinion.
Patients with MAFLD without fibrosis can be monitored at intervals of 2–3 years in the absence of worsening metabolic risk factors using a combination of non-invasive liver scores (eg NFS and FIB-4) and liver stiffness measurement by elastography in primary care.12,19 Patients with MAFLD and fibrosis (especially those with stage 2 fibrosis, pre-cirrhosis or cirrhosis) should have the benefit of non-GP specialist consultation, which will likely determine the long-term monitoring schedule on the basis of individual patient characteristics.11 Patients with cirrhosis should undergo six-monthly surveillance for hepatocellular carcinoma with abdominal ultrasonography and alfa-fetoprotein measurements, and all will require endoscopy at some stage to look for and manage varices, especially patients with a platelet count <150 × 109/L.11 Bone health and nutrition are additional aspects that require close attention in those with cirrhosis, either through the primary practitioner or a non-GP specialist.11
Conclusion
MAFLD is a consequence of metabolic dysregulation that is increasing in prevalence, with increasing recognition of ‘lean MAFLD’. All individuals with metabolic risk factors should be screened with abdominal ultrasonography, liver elastography and non-invasive liver scores. Management of MAFLD should be focused on lifestyle programs that reduce weight through improved dietary composition and increased physical activity.
Key points
- MAFLD is a consequence of metabolic dysregulation resulting in increased fat deposition in the liver.
- MAFLD can develop in lean patients, and patients with metabolic risk factors should be screened.
- Screening for hepatic steatosis should be performed with abdominal ultrasonography; if available, additional assessment with liver elastography and non-invasive liver scores should be considered.
- Management of MAFLD currently focuses on weight loss, an improved dietary composition (similar to a Mediterranean diet composition) and increased physical activity.
- There is no current specific pharmacological treatment for MAFLD; however, there are numerous ongoing clinical trials.