This article is the sixth in a series of articles on important topics in neurology.
Parkinson’s disease is a neurodegenerative disorder characterised by slow disease progression over many years and often conceptualised as a disease of three stages – early, mid/advanced and late stage. Dopaminergic oral medications may adequately control symptoms in early Parkinson’s disease before the development of motor fluctuations of the mid/advanced stage. Late-stage Parkinson’s disease (LSPD) is a syndrome of frequent falls, cognitive impairment, visual hallucinations and need for residential care.1,2 While many symptoms remain levodopa responsive throughout all disease stages, levodopa-unresponsive, predominantly non-motor symptoms come to the fore in LSPD. Although a chronic disease, patients with LSPD commonly experience episodes of acute clinical deterioration in the community setting. The general practitioner (GP) is typically the first clinician to evaluate such patients and is faced with multifaceted challenges – what has caused the deterioration? Is polypharmacy a factor – if so, what can be altered safely? What additional medications may be useful, and which should be avoided? Is emergent hospital referral appropriate? Here, we provide our approach to such questions based on current evidence and experience. We explore acute changes in non-motor symptoms – spanning pain, neuropsychiatric and cognitive states – and acute decline in motor symptoms, such as a significant fall and loss of mobility.
Individuals with LSPD will typically still access active medical treatment. However, recurrent acute deteriorations – in particular aspiration pneumoniae, falls with fractures, sepsis and severe pressure ulcers – portend a poor prognosis and may herald the terminal stage of Parkinson’s disease.3 GPs, with their invaluable GP–patient–carer relationship and prior discussions with the patient and family regarding treatment goals, are ideally placed to decide the appropriate level of intervention. While comorbidity in such a population will undoubtedly influence management decisions, there are a number of key aspects that should not be overlooked in LSPD, which may supplement the holistic care provided by GPs.
Acute non-motor deterioration
The confused patient: Delirium versus dementia
Eighty-three per cent of those who survive 20 years following a Parkinson’s disease diagnosis will experience dementia associated with Parkinson’s disease (PDD).4 PDD is characterised by the gradual emergence of day-to-day fluctuations in cognition, confusion, attention and alertness, as opposed to the persistent amnestic deficits of Alzheimer’s disease. It is important that GPs are alert to the higher risk of delirium in patients with LSPD,5 particularly in those with PDD, and the inherent morbidity (prolonged hospital stay, cognitive and motor decline) and mortality.6 Consider the common scenario of an elderly nursing home resident with Parkinson’s disease who exhibits increased paranoid delusions, resulting in refusing of meals and medications. Rigidity may increase from acute withdrawal from dopaminergic medication, constipation may worsen if aperients are declined and oral intake is reduced, and psychotic symptoms may be exacerbated. Dopaminergic medications can predispose to or exacerbate delirium, whereas neuroleptic medication commonly used to treat delirium in older adults can worsen the motor symptoms of Parkinson’s disease, resulting in increased rigidity, bradykinesia, falls, impaired swallowing, aspiration and other complications.5
An acute change (hours to days) in consciousness, attention or cognition, or new perceptual disturbance, should prompt consideration of delirium. It can be tempting to attribute the acute deterioration to Parkinson’s disease itself, particularly as many features of the latter overlap with those of PDD, such as day-to-day fluctuations in cognition or alertness. Delirium is frequently accompanied by emotionally upsetting tactile and auditory hallucinations, which may help distinguish delirium from the chronic visual hallucinations and illusions of PDD.7
Delirium aetiologies pertinent to Parkinson’s disease: Constipation, polypharmacy, infection, pain
Addressing the underlying cause is the first line of management (Figure 1). In these authors’ experience, constipation is frequently overlooked by patients and carers but can have a surprisingly significant negative impact on cognitive state in all stages of Parkinson’s disease and may take a number of weeks to adequately treat (Table 1). Polypharmacy (or recent change) and infection are well-known precipitants of delirium. Medications that can potentially cause or exacerbate delirium should be identified and withdrawn.8 Commonly implicated psychoactive medications include anticholinergics, opioids, benzodiazepines and non-benzodiazepine sedatives (eg the ‘zolpidem-like’ medications).
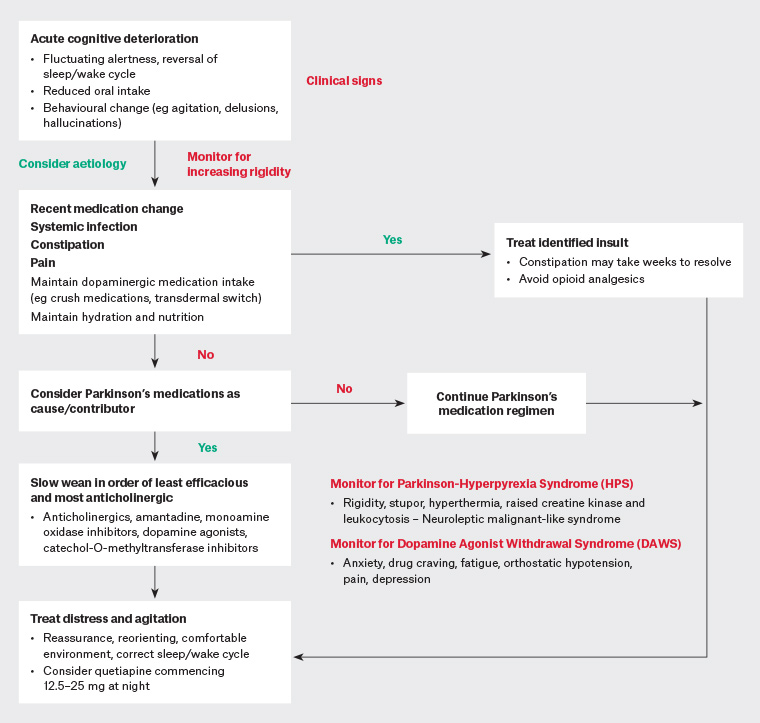
Figure 1. An approach to assessing and treating acute neuropsychiatric deterioration in a patient with late-stage Parkinson’s disease. Click here to enlarge.
Parkinson’s disease medications may contribute to delirium and should also be reviewed. Expert consensus is that if Parkinson’s disease medications are suspected to be contributing to psychotic symptoms, medications with the least antiparkinsonian and greatest anticholinergic effect should be withdrawn first.9,10 Medications should be withdrawn in the following order: anticholinergics, amantadine, monoamine oxidase inhibitors (rasagiline, selegiline, safinamide), dopamine agonists (pramipexole, rotigotine), catechol-O-methyltransferase inhibitors (entacapone), followed by levodopa as a last resort. Oral levodopa is rarely the sole cause of delirium in patients with Parkinson’s disease, and the dose should be lowered with extreme caution and only after failure of the preceding measures. Abrupt cessation of dopaminergic medication can both exacerbate delirium and lead to a neuroleptic malignant-like syndrome (parkinsonism–hyperpyrexia syndrome) characterised by marked rigidity, stupor, hyperthermia, raised creatine kinase and leukocytosis (discussed in greater detail later in this article). We urge caution in attributing such a presentation solely to infection.
While non-pharmacological management strategies – such as providing a stable and comfortable environment, maintaining hydration and nutrition and correcting the sleep–wake cycle – remain first line, neuroleptic medications are often required to manage distressing psychotic symptoms or behavioural disturbance. Quetiapine remains a relatively safe option for those in whom pharmacotherapy is required (eg quetiapine 12.5–25 mg at night, titrating in similar increments up to 50 mg twice daily). Parkinson’s disease–associated delirium may take 4–6 weeks to resolve despite removal of the precipitant.5 More severe agitation or psychotic deterioration unresponsive to quetiapine may require specialist psychiatric advice or review.
Pain
Moderate-to-severe pain is an unusual, isolated manifestation of Parkinson’s disease, particularly as an acute phenomenon. However, pain can precipitate acute behavioural or motor change in LSPD. In such instances, an alternative, frequently multifactorial, systemic, musculoskeletal, neuropathic or radicular cause should be sought. Patients may not volunteer this symptom, so it is important to screen specifically for pain. Given that patients with LSPD are frequently sensitive to analgesics, a clear diagnosis is paramount and should dictate specific treatments, such as targeted physiotherapy for painful adhesive capsulitis, root injection in radiculopathy, pressors for ‘coat-hanger’ headache and neck/shoulder pain of orthostatic hypotension, and aperient regimens for constipation-related abdominal discomfort (all common complications seen in patients with Parkinson’s disease; Table 1). Once such causes are excluded, dystonic, akathisic and/or central parkinsonian pain warrant consideration.11 Discomfort from dystonia, rigidity and akinesia may improve dose to dose with levodopa. Optimising dopaminergic stimulation is important for such clearly dystonic or akinetic pain and may alleviate the nocturnal discomfort of restless legs syndrome.
Troubleshooting other non-motor symptoms is presented in Table 1.
| Table 1. The treatment of acute complications of late-stage Parkinson’s disease |
| Symptom |
Treatment options |
Agitation/behavioural change
|
Non-pharmacological
- Environmental optimisation, reassurance
Pharmacological
- Quetiapine 12.5–25 mg at night initially
|
| Hallucinations |
Non-pharmacological
- Exclude contributing factors such as hearing and vision defects, infection, constipation, metabolic and electrolyte disturbance
Pharmacological (if distressing/loss of insight)
- Avoid exacerbating medications such as opiates
- Consider weaning anti-parkinsonian medications (order: refer to main text; levodopa weaning as last resort)
- Quetiapine 12.5–25 mg at night titrating up to 50 mg twice daily
- Clozapine – prescribed by specialist (Clopine Central registered) prescribers – avoid dopamine blockers
|
Constipation
|
- Dietary fibre and hydration
- Aperients
- Bulk forming (fibre supplements)
- Osmotic (regular macrogol, 1–2 sachets twice daily)
- Stimulant (short-term use only)
- Softeners (regular docusate sodium)
- Probiotics
- Enemas, such as sodium phosphate or sodium citrate/sodium alkylsulfoacetate
|
| Orthostatic hypotension (fall in blood pressure of >20 mmHg systolic and/or >10 mmHg diastolic after two minutes of standing from a seated or lying position) |
Non-pharmacological
- Hydration – at least 1.5 L/day
- Increase salt intake
- Compression stockings
- Slow change in posture; head up tilt of bed at night 30–40°
- Avoid large meals, warm environment
Pharmacological
- Short-acting pressors: fludrocortisone 100–300 µg in divided doses morning and midday, +/– midodrine 2.5–10 mg; avoid evening administration because of risk of supine hypertension
- Pyridostigmine 30–60 mg three times daily
- Avoid exacerbating medications if possible (ie diuretics, nitrates, antihypertensives)
- Schedule pressor prior to levodopa to mitigate hypotensive effect
|
| Neuropathic pain* |
- Amitriptyline (low dose, benefit sleep and urinary symptoms. May exacerbate constipation or confusion at higher doses)
- Duloxetine (may also aid mood and insomnia)
|
| Dystonia, akinesia, rigidity |
- Optimise dopaminergic therapy, trial safinamide
|
| *Reaching a precise diagnosis is a vital first step. |
Acute motor deterioration and falls
The motor fluctuations of advanced-stage Parkinson’s disease may paradoxically become less prominent with progression to LSPD, when motor disability from severe akinesia without rigidity, frequent freezing of gait and postural instability may lead to wheelchair dependency.12 The majority of motor symptoms remain levodopa responsive, but the peak benefit declines over time.1,13 Simply increasing medications to achieve the same benefit can result in intolerable side effects, and a nuanced approach is required (Table 2).
| Table 2. Troubleshooting motor symptoms according to the ‘ON’, ‘transition’ and ‘OFF’ phases, typical levodopa response (induced, responsive or resistant) and potential strategies |
| Motor symptom |
Description |
Levodopa response |
Strategies |
| ON |
| Peak-dose dyskinesia |
Involuntary movements within 30–90 minutes of levodopa dose |
Induced |
- Consider intervention if bothersome (patient may prefer over OFF)
- Lower oral adjuncts or levodopa dose
- Device-assisted therapies*
|
| Transition phase |
| Diphasic dyskinesia |
Typically leg-predominant ballism or dystonia between ON and OFF state |
Variable |
- Avoid protein/meals with medication
- Exclude gastroparesis or constipation
- Reschedule timing of medication
- Increase dose*
- Consider adjuncts*
- Device-assisted therapies*
|
| OFF |
| Wearing off |
Re-emergence of parkinsonian symptoms near end of dose interval |
Responsive |
- Refer to ‘Diphasic dyskinesia’
|
| Dystonia |
Involuntary agonist and antagonist muscle co‑contractions: abnormal postures or repetitive movements, typically affecting the foot |
Responsive |
- Evening controlled-release formulation levodopa for morning pre-medication dystonia
|
| Dose failure |
Failure of parkinsonian symptoms to respond to dose |
Unresponsive |
- Refer to ‘Wearing off’
- Exclude use of dopamine antagonists such as antihistamines, antiemetics
|
| Freezing of gait |
Impairment or cessation of forward mobility of legs +/– persistent truncal forward movement, often leading to falls with ‘feet stuck to the ground’ |
Responsive |
- Avoid precipitants such as narrow spaces
- Cues such as shifting weight, lifting the knees, visual aids to step over
- Physiotherapy
|
| Tremor |
Low-frequency, low-amplitude rest tremor; re‑emergent tremor on action also common |
Responsive |
- ‘Load’ the limb, for example by using weighted cutlery
- Relaxation techniques if anxiety a contributor
- Device-assisted therapies*
|
| *Consult specialist neurology services |
In cases of acute akinesia and rigidity, we typically adhere to first principles and consider other causes, including the emergencies of acute central nervous system insults such as stroke, subdural haemorrhage or spinal cord compression (it is important to be alert to acute exacerbation of chronic degenerative compressive cervical myelopathy from a fall) and medication errors.
Patient report of an acute change in medication response should prompt a probing history, including collateral. Confounders masquerading as medication unresponsiveness include poor compliance and medication errors due to cognitive impairment, impaired absorption due to constipation, or timing of high-protein meals within one hour of medication administration. Acute akinesia should also prompt a review for an acute precipitant, such as infection, inadvertently consumed dopamine-blocking medications (eg metoclopramide, prochlorperazine, promethazine, olanzapine, risperidone, haloperidol) or delirium. In patients who have undergone deep brain stimulation (DBS), the implantable pulse generator (IPG) should be interrogated to ensure it is still ‘on’ and has an adequate battery level. The IPG is typically found on the right anterior chest wall. The IPG site, DBS wire course (subcutaneously in right lateral posterior neck and scalp) and scalp surgical site should be assessed for signs of infection. Patients should routinely check the battery level monthly with their programmer. If patients or carers are unfamiliar with this, then their DBS team can guide them through this process via telehealth. If the DBS was inadvertently switched off, it should be turned on again immediately and the DBS team updated urgently. The IPG may need to be replaced emergently, and the patient is at risk of parkinsonism–hyperpyrexia syndrome.
Parkinsonism–hyperpyrexia syndrome is a rare neurological emergency, but it has a high mortality and clinical features similar to neuroleptic malignant syndrome. It is characterised by a sequence of increasing rigidity progressing to acute akinesia and near-complete immobility, hyperpyrexia, confusion/obtundation, autonomic instability, diaphoresis with elevated creatine kinase and leukocytosis. Respiratory distress and seizures may occur. It is most often precipitated by withdrawal, or rapid tapering, of Parkinson’s disease medications or DBS malfunction.14,15 Other precipitants include a hot environment, dehydration, constipation, infection and dopamine-blocking medications. First symptoms occur hours to days after medication alteration. Early recognition can be life saving. Initial management involves hydration, antipyretic medications, cooling measures and reintroduction of Parkinson’s disease medications (temporary patch formulation, subcutaneous apomorphine or nasogastric tube may be required) along with urgent referral to acute hospital services.
Patients with LSPD are at increased risk of serotonin syndrome, which may precipitate a sudden decline in mobility, increased rigidity, altered mental status and hyperreflexia in addition to autonomic hyperactivity. It is worth noting that the risk of serotonin syndrome from co-prescription of monoamine oxidase inhibitors and low-moderate dose-selective serotonin/norepinephrine reuptake inhibitors is low and tolerated without adverse effects in the majority of patients. Mild symptoms are easily overlooked, with tremor, rigidity or diarrhoea misattributed to Parkinson’s disease itself. Most symptoms of serotonin syndrome develop within hours of exposure to offending agents. The timeline of symptoms, diarrhoea and predominant lower limb findings may aid in distinguishing serotonin syndrome from parkinsonism–hyperpyrexia syndrome in more severe cases. A clinical suspicion of serotonin syndrome should prompt review of all serotonergic medications, including recent additions or dose increases of medications such as opioids, pregabalin, anti-emetics, some herbal supplements (eg St John’s wort, ginseng) and triptans, and consideration of emergent referral for hospital-based treatment in moderate-to-severe cases.
Falls
Falls are a common occurrence in LSPD but are often underreported because of a fear of entering residential care.15 Key factors in LSPD include perturbations in balance, freezing of gait (FoG), attentional fluctuations and orthostatic hypotension.16,17 FoG is one of the most common reasons for falls but often goes unrecognised by patients. Patients tend to fall forwards on initiation or turning and tremble in place. Precipitants may include tight spaces or doorways (Table 1). Orthostatic hypotension may cause falling within minutes after standing. Some, but not all, patients experience warning light-headedness, visual dulling, diaphoresis, global weakness or ‘coat-hanger’ distribution pain (for treatment refer to Table 1). We also advise consideration of common comorbid causes of ‘going off my legs’ including neuropathy, myelopathy, myopathy and pain (eg arthralgia, radiculopathy and spinal or vascular claudication).
Finally, it is important to remember that hypophonia and dysphagia are also motor symptoms that may be levodopa responsive, particularly the voluntary oral preparatory phase. Patients will deteriorate when medications are inadvertently missed or withheld as a result of impaired swallowing, leading to a vicious cycle of worsening dysphagia. Dispersible tablets or crushing non–modified release tablets (Table 3), short-term insertion of a nasogastric tube to facilitate administration or temporary usage of a levodopa-dose equivalent rotigotine patch (starting at 2–4 mg/24 hours and titrating up slowly in agonist-naive patients, not exceeding 8 mg/24 hours without non-GP specialist input) should be implemented until resumption of regular oral intake.
| Table 3. Strategies for administration of commonly prescribed medications if swallowing oral tablets becomes problematic in late-stage Parkinson’s disease |
| Medication |
Strategy |
Levodopa/benserazide
Levodopa/carbidopa
Levodopa/carbidopa CR |
May be crushed using pill crusher
Switch to dispersible version |
Rasagiline (1 mg)
Selegiline (5 mg) |
May be crushed using pill crusher |
| Entacapone (200 mg) |
May be dissolved but with reduced clinical efficacy |
| Pramipexole |
May be crushed using pill crusher
Consider switch to transdermal rotigotine (patch)
If taking ER preparation, switch to regular-release formulation, crush and divide doses throughout the day
|
| Levodopa/carbidopa/entacapone |
Source entacapone and levodopa/carbidopa separately and follow strategy above |
| Amantadine |
Capsules – cannot be crushed
Consider switch to oral liquid (available through Special Access Scheme) |
| CR, controlled release; ER, extended release |
Other resources and referral to hospital
The decision to refer a patient with LSPD experiencing an acute clinical deterioration to hospital is an individualised one. GPs are well placed to evaluate the nature and severity of the insult, the probability of reversibility (if a clear diagnosis exists), the patient’s new care needs, their underlying health and their philosophy regarding their care in LSPD, in the context of the patient’s setting (residential versus home) and resources available (eg mobile imaging and pathology; in-reach geriatric care teams; and telehealth with specialist services including neurology, community palliative services and specialist nursing where device-assisted therapies are in use). Such decisions are weighed against the risk of delirium with a change in environment and a likely prolonged admission. A delirium with a reversible cause is clearly best managed in their home environment where feasible. Serious injuries, such as suspected fractures, warrant hospital assessment. A fall, particularly if unwitnessed, and acute changes such as agitation, reduced alertness, appearance of discomfort, nausea, vomiting or focal neurological deficit also requires urgent referral. Where feasible, it is preferable for a patient to be referred to a hospital where they are known to specialist neurology/geriatric services to optimise continuity of care.
GPs, with their invaluable GP–patient–carer relationship and insights into previously expressed wishes, are ideally placed to make critical decisions regarding treatment goals and the appropriate level of intervention. While well, we advise patients to be aware of their options to access advanced care directives (state and territory specific) and medical enduring power of attorney. It is worth re-visiting advanced care directives with patients periodically, as perspectives on care may change with burden of disease, life events or novel treatments. Recurrent acute deteriorations – in particular aspiration pneumoniae, falls with fractures, sepsis and severe pressure ulcers – portend a poor prognosis and may herald the terminal stage of Parkinson’s disease.3 The decision to transition from active treatment to comfort and supportive measures may be appropriate with input from patients, family members and carers. The patient’s specialist neurologist or geriatrician may provide further support in this decision, but carefully broached discussions with patient and carers in advance of such acute deterioration are critical to inform approach at times of crises. Palliative care services (depending on local availability) may facilitate transfer to a hospice, engage directly or provide advice.
Conclusion
GPs are typically the first port of call for acute deterioration in a patient with LSPD. Excluding a systemic cause for a motor or non-motor deterioration is always a sensible initial approach, with polypharmacy remaining a common pitfall; however, levodopa itself is a rare cause of delirium. Seeking support from outreach specialist neurology, geriatric or palliative services may prove useful in avoiding lengthy distressing hospital admissions.