Case
A man aged 88 years was referred to a geriatric clinic for falls evaluation. He had worsening postural instability over the past three years with multiple unprovoked falls. His medical history included benign prostate hypertrophy and hypertension with no other vascular risk factors. He described his falls as if being pulled backwards by a magnetic force. On examination, he had a flat affect with monotonous voice. He also had bilateral bradykinesia and cogwheel rigidity with significant axial rigidity. Pull test was positive. He had presence of primitive reflexes including a positive glabella tap and palmomental reflex. His vertical gaze was restricted, particularly his downward gaze. This was overcome by oculocephalic manoeuvre. Executive dysfunction was evident on cognitive assessment.
Relevant negative findings included absence of tremor, autonomic dysfunction and cerebellar signs. Speech and language were preserved. There was no behavioural disturbance. The patient had not been taking antidopaminergic medications. Cerebral magnetic resonance imaging (MRI) revealed midbrain atrophy (Figure 1). There was no significant vascular disease, iron deposition in the substantia nigra nor hydrocephalus. A diagnosis of probable progressive supranuclear palsy (PSP) with predominant parkinsonism was made on the basis of the Movement Disorder Society (MDS) criteria.1 Given the clinical suspicion of PSP, the decision was made against a trial of levodopa as the risk of harm secondary to orthostatic hypotension outweighed potential benefit. Over the next year, the patient had repeated hospitalisations for falls and was deemed unsafe to return home.
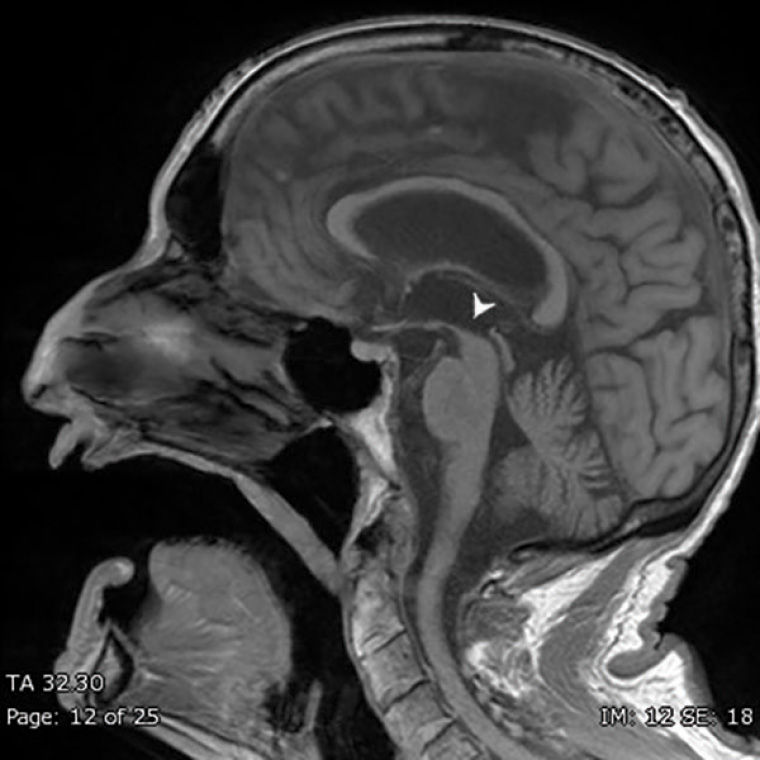
Figure 1. T1-weighted sagittal view magnetic resonance imaging showing midbrain atrophy, also known as the Hummingbird sign
Question 1
What is atypical parkinsonism?
Question 2
What is PSP?
Question 3
How is PSP diagnosed?
Question 4
How is PSP managed and what does it mean for the patient?
Answer 1
Not all parkinsonism is idiopathic Parkinson’s disease. Parkinsonism can be typical or atypical. Atypical parkinsonism is a group of hypokinetic rigid disorders with specific features, distinguishing it from typical parkinsonism. Common atypical parkinsonism includes PSP, multiple system atrophy (MSA) and corticobasal syndrome (CBS). The salient clinical signs to differentiate these disorders are described in Tables 1 and 2. Hallmarks of atypical parkinsonism include early and recurrent falls, autonomic instability, early onset of cognitive impairment, cerebellar signs and levodopa resistance. It is an under-recognised cause of falls in the elderly.2 It is important to distinguish between idiopathic Parkinson’s disease and atypical parkinsonism, as atypical parkinsonism carries a poorer prognosis with more rapid progression and limited availability of effective pharmacological therapy.
| Table 1. Features of typical versus atypical parkinsonism2 |
| Features |
Typical parkinsonism |
Atypical parkinsonism |
| Tremor |
Rest tremor predominant |
May be present but not predominant feature |
| Symmetry of hypokinesia |
Asymmetrical |
Symmetrical |
| Response to levodopa |
Good response |
Poor response |
| Atypical features |
Absent |
Present (for further details, refer to Table 2) |
| Table 2. Common atypical parkinsonism disorders2 |
| Disorder |
Clinical features |
| Progressive supranuclear palsy |
- Vertical gaze palsy (downward > upward)
- Falling backwards
- Axial rigidity (axial > limbs)
- Dysarthria, dysphagia
- ‘Astonished facies’ – reduced blinking, eyelid apraxia
|
| Multiple system atrophy |
- Autonomic features: orthostatic hypotension, urinary incontinence
- Cerebellar signs
|
| Corticobasal syndrome |
- Dystonia, typically of one limb
- Alien limb phenomenon
- Ideomotor apraxia – inability to make a purposeful movement in response to verbal command or imitation
|
Answer 2
PSP is a neurodegenerative disorder formerly referred to as Steele–Richardson–Olszewski syndrome that is characterised by tau protein aggregation, an element also seen in Alzheimer’s disease.3 It is the most common variant of atypical parkinsonism, with an incidence of 5–6 per 100,000.4 The subcategories of PSP, also known as clinical predominance types, are indicative of its diverse manifestation. They include parkinsonism, oculomotor dysfunction, postural instability and gait freezing, frontal presentation, speech disorder and corticobasal syndrome. The most recognised type is the Richardson syndrome.
Answer 3
As with many neurodegenerative disorders, specific biomarkers are yet to be identified. Diagnosis is made on the basis of the MDS criteria, which consist of core features including oculomotor dysfunction, postural instability, akinesia and frontal cognitive dysfunction. Vertical supranuclear gaze palsy, in which the patient is unable to generate voluntary vertical saccades but is overcome by ocular reflexes induced by head movements, is highly suggestive of PSP. Supportive features include predominant midbrain atrophy on neuroimaging, also known as the Hummingbird sign (although not always seen and often a late finding), and levodopa resistance. It is worth noting that initial response to levodopa may be seen, particularly in PSP-parkinsonism, the second most common subtype of PSP, but this response wanes promptly. In this case presentation, the patient met core features of PSP with vertical gaze palsy, axial rigidity and mild cognitive impairment.5 In addition, the patient’s MRI (Figure 1) further supported the diagnosis of PSP.
Answer 4
Disease progression in PSP is usually more rapid than in idiopathic Parkinson’s disease, with an average life expectancy of seven years from diagnosis.6–8 Early dysphagia, cognitive impairment and older age at onset have been proposed as poor prognostic indicators.8 Management remains supportive. Symptomatic pharmacological therapy, such as levodopa, could be considered for bradykinesia and rigidity; however, the majority of patients are levodopa resistant. Ideally, advance care planning should be introduced early in the course of the disease. A multidisciplinary approach is crucial to prolonging independence, preventing falls and preserving quality of life. This includes physiotherapy and occupational therapy involvement. Speech therapy referral for swallowing assessment and strategy to mitigate risk of aspiration should be considered. Clinical trials involving tau-targeted therapy and gene therapy are actively being researched.9,10
Key points
- Not all parkinsonism is idiopathic Parkinson’s disease.
- Atypical parkinsonism is an important differential in falls evaluation in the elderly.
- PSP has a more rapid disease progression and poorer prognosis than idiopathic Parkinson’s disease.