Bronchiectasis is a condition in which the airways are dilated with increased sputum production, recurrent exacerbations and impaired quality of life. The estimated prevalence of bronchiectasis is at least 1470/100,000 in central Australian Aboriginal children.1 There are limited reported data on the prevalence of bronchiectasis in Australia; however, the Australian Bronchiectasis Registry (ABR) has been addressing this by recruiting patients since 2015. The data collected between March 2016 and August 2018 were first reported in 2019, when the ABR identified 589 individuals with bronchiectasis from six tertiary centres across New South Wales and Queensland.2 Chronic respiratory disease is a significant burden in the Australian Aboriginal community, particularly in the Northern Territory.3 A significant proportion of Aboriginal patients with bronchiectasis have bronchiectasis in combination with chronic obstructive pulmonary disease,4 and some patients have restricted lung disease. General practitioners (GPs) are often the first point of medical contact for patients with bronchiectasis and have a crucial role in managing and maintaining their long-term respiratory function and quality of life.
Bronchiectasis is often associated with pulmonary restriction either secondary to pulmonary fibrosis or poor lung growth in childhood. The vicious cycle hypothesis describes a phenomenon in which the airway distortion in bronchiectasis impairs mucociliary clearance, therefore causing persistent bacterial colonisation and airway inflammation that ultimately leads to airway destruction.5–7 Conditions that are associated with bronchiectasis include cystic fibrosis, primary ciliary dyskinesia, rheumatic and autoimmune diseases, pulmonary infections, allergic bronchopulmonary aspergillosis and Young syndrome (Box 1).8 As a result, these groups of patients often have recurrent exacerbations, frequent presentations to health services and impaired quality of life.
The aim of this article is to provide a comprehensive summary of the important features to recognise in bronchiectasis and an updated guide to the management of bronchiectasis.
| Box 1. Aetiologies of bronchiectasis8 |
- Pulmonary infections (including tuberculosis)
- Cystic fibrosis
- Primary ciliary dyskinesia
- Rheumatic disease
- Autoimmune diseases
- Allergic bronchopulmonary aspergillosis
- Young syndrome
|
Recognising symptoms
Chronic cough and sputum production over a span of months to years is the primary symptom of bronchiectasis. Other associated symptoms include dyspnoea, occasional haemoptysis, dry cough or pleuritic chest pain, although these are less common. Patients may report recurrent episodes of infection-driven exacerbations that have been previously managed with courses of antibiotics.9,10
Investigation and diagnosis
The workup for bronchiectasis consists of establishing the diagnosis radiologically, documenting disease severity by symptoms and spirometry and performing investigations for underlying and associated diseases (Table 1).11
| Table 1. Summary of recommended investigations for patients with suspected bronchiectasis11–14 |
| Pathology |
- Full blood examination
- Biochemistry
- C-reactive protein
- Aspergillus serology
- Autoimmune screen
- Immunoglobulin classes G, A, M and E
|
| Sputum microscopy and culture |
- Bacteria
- Mycobacteria
- Nontuberculous mycobacterium
|
| Functional assessment |
- Spirometry
- Diffusing capacity for carbon monoxide
- Six-minute walk test
|
| Imaging |
- Chest X-ray
- High-resolution computed tomography of the chest
- Early discussion with paediatrician if computed tomography in a child is considered
|
| Additional tests in discussion with non–general practitioner specialist |
- Sweat test
- Extended cystic fibrosis transmembrane conductance regulator gene mutation
|
Imaging
Chest radiography is the initial imaging modality of choice to exclude other causes of patients’ respiratory symptoms and examination findings.11 An early discussion with a paediatrician is strongly recommended if a computed tomography scan is considered in a child. High-resolution computed tomography (HRCT) is the modality of choice. The characteristic findings are dilatation of the airways (larger in cross-sectional size than their accompanying blood vessel) that appears as parallel (tram) lines or end-on ring shadows with a lack of tapering.12 The tree-in-bud pattern may be noted on HRCT; this represents small airways that are affected and appears in the periphery with irregular, short (2–4 mm) linear branching markings. The airways may contain mucopurulent plugs or debris with post-obstructive air trapping. Persistent findings of tree-in-bud changes on imaging are particularly suspicious for nontuberculosis mycobacterial infection (Figure 1).13
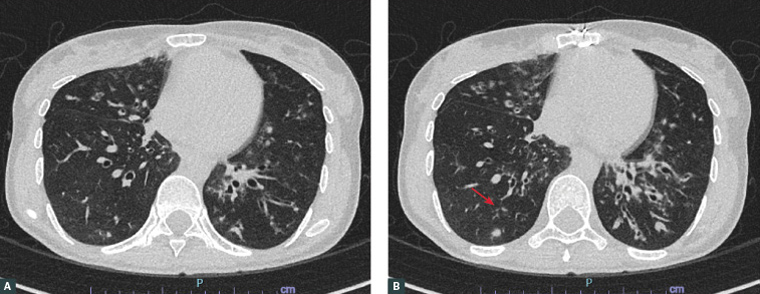
Figure 1A and B. Axial computed tomography scan of the chest demonstrating dilated airways on bilateral lobes of the lungs. Image B demonstrates the lower part of the lungs when compared with image A. Dilated airway changes are more pronounced on the left lung in image B. Note tree-in-bud features on the right lung lobe (arrow).
Pathology
At the time of diagnosis, every patient with bronchiectasis should have the following investigations: full blood examination, biochemistry, C-reactive protein, aspergillus serology, autoimmune screen and immunoglobulin (Ig) levels, such as IgG, IgA, IgM and IgE. Sputum should be sent for microscopy and culture for bacteria, mycobacteria and nontuberculous mycobacteria.14 In all children and selected adults, a sweat test and extended cystic fibrosis transmembrane conductance regulator gene mutation testing can be considered after a discussion with the respiratory specialist. These selected adults comprise patients with episodes of bowel obstruction, heat prostration, pancreatitis, pre-existing liver disease, male infertility or age <50 years.14
Functional assessment
Lung function testing and a six-minute walk test should be performed for the purpose of functional assessment in patients with bronchiectasis. The lung function of patients with bronchiectasis can be normal, obstructed, restricted or a combination of obstruction and restriction.Obstructive spirometry is demonstrated by a reduction in forced expiratory volume in the first second, and patients may also have a reduction in their forced vital capacity.11
Referral to non-GP specialists
It is recommended that patients with newly diagnosed bronchiectasis be referred to a multidisciplinary team consisting of respiratory specialists, physiotherapists and dietitians. These patients often have underlying causes that require longer-term management, including cystic fibrosis, allergic bronchopulmonary aspergillosis or recurrent exacerbations that are difficult to manage. Respiratory specialists may consider flexible bronchoscopy if there is suspicion of mycobacteria or a focal obstructive lesion. Patients who have underlying rheumatoid or autoimmune conditions should be referred accordingly to the respective specialty for further management of the primary diagnosis.
Management of bronchiectasis
The management of bronchiectasis comprises both non-pharmacological and pharmacological approaches (Table 2). The non-pharmacological approach is aimed at effective sputum clearance and opening the airways with chest physiotherapy. The pharmacological approach can be broadly categorised into treatment of acute exacerbations, sputum clearance and reduction of recurrent exacerbations.
| Table 2. Summary of recommended management strategies15–29 |
| Non-pharmacological management |
Pharmacological management |
- Chest physiotherapy
- Bubble-positive expiratory pressure
- Avoidance of lung irritants (eg chemicals at occupational areas, smoking and vaping)
- Optimisation of nutritional status
- Pulmonary rehabilitation
|
- Up-to-date immunisations
- Acute exacerbations
- 14 days of oral antibiotics, which can be tailored to a specific organism if sputum cultures are positive with sensitivities
- Referral to emergency department if hypoxic or have not responded to course of oral antibiotics
- Long-term therapies
- Long-term antibiotics with macrolides (azithromycin and erythromycin)
- Mucolytics
- Inhaled corticosteroids or regular long-acting bronchodilators in patients with underlying chronic obstructive pulmonary disease or asthma
- Inhaled antibiotics
|
Non-pharmacological therapies
Effective chest physiotherapy has been shown to improve sputum clearance and reduce frequent exacerbations.15,16 There are various airway clearance techniques available to facilitate loosening of viscid secretions mechanically. In particular, patients who performed bubble-positive expiratory pressure (bubble PEP) were able to expectorate a greater amount of sputum when compared with the control group at all times and the group who performed the active cycle of breathing technique at 60 minutes post active cycle of breathing technique. Bubble PEP is a device used for sputum clearance in patients who have daily sputum production.17
Effective sputum clearance results in an improved quality of life and reduction of cough. A Cochrane review by Lee et al showed that positive expiratory pressure therapy offered comparable effects on cough-related qualify of life, symptoms of breathlessness and health-related quality of life (HRQoL) when compared with other airway clearance techniques.16 However, irrespective of clinical disease state, short- and medium-term use of PEP therapy has no greater effect on sputum clearance than other types of techniques. There is no evidence that PEP improves lung function.16
Other documented techniques include slow expiration with the glottis opened in the lateral posture (ELTGOL). A study showed that ELTGOL performed twice daily for one year improved sputum clearance in patients with bronchiectasis, resulting in a reduced number of exacerbations, reduced cough impact and improved quality of life.15
Avoidance of lung irritants, including chemicals at occupational areas, smoking and vaping, is advisable to all patients. The nutritional status of patients with bronchiectasis should be optimised, as studies have found that a lower body mass index is associated with increased exacerbations, chronic colonisation by Pseudomonas aeruginosa and poorer outcomes when compared with a normal body mass index.18,19 Referral to pulmonary rehabilitation can assist patients with improving exercise capacity and HRQoL.20–23
Pharmacological management
Medical management encompasses management of acute exacerbations and long-term therapies with bronchiectasis action plans to facilitate sputum expectoration and reduce recurrent exacerbations. It is recommended that all patients are vaccinated against COVID-19, influenza and pneumococcal diseases.
Acute exacerbations
With acute exacerbations, bronchiectasis should be managed with 14 days of oral antibiotics. This can be tailored to a specific organism if sputum cultures are positive with sensitivities; therefore, early testing of sputum cultures is strongly recommended. This practice is based on expert consensus and is recommended in the European Respiratory Society (ERS) guidelines.24,25 Patients who have new isolation of P. aeruginosa should be offered eradication antibiotic treatment.24 This is performed with the intention to achieve complete clearance of P. aeruginosa from the airways and may require up to a three-month antibiotic regimen. A pooled analysis performed by the ERS found that there are potential benefits comprising reduced frequency of exacerbations, improved quality of life and negative sputum samples post eradication of P. aeruginosa; however, this evidence is indirect.24 Patients who are hypoxic or have not responded to oral antibiotics should be referred to the emergency department for further management with intravenous antibiotics and supplemental oxygen support as required.
Long-term management
Long-term antibiotic treatment with macrolides such as azithromycin or erythromycin should be considered for patients who have three or more exacerbations per year.24 This has been found to reduce the frequency of infective exacerbations in patients with bronchiectasis and is well tolerated.26 To improve sputum clearance, long-term mucolytics (≥3 months) should be offered to patients with poor quality of life who have difficulties expectorating sputum and have failed to effectively expectorate sputum using standard airway-clearance techniques.24
Inhalers
Patients do not require inhaled corticosteroids or regular long-acting bronchodilators unless there is underlying chronic obstructive pulmonary disease or asthma. Bronchodilators, inhaled mucoactive medications and inhaled antibiotics can be used prior to physiotherapy sessions to increase tolerability and optimise pulmonary deposition in diseased areas of the lungs.24
Inhaled antibiotics
A recent study has shown that inhaled antibiotics are well tolerated and can reduce bacterial load and achieve a small but statistically significant reduction in frequency of exacerbations. However, they have not been shown to result in clinically significant improvements in the quality of life of patients.27 ORBIT-3 and ORBIT-4 were international, double-blind, randomised, placebo-controlled phase 3 trials looking at the safety and efficacy of inhaled liposomal ciprofloxacin in patients who have non–cystic fibrosis bronchiectasis with chronic P. aeruginosa lung infection. In ORBIT-4, it was found that patients have longer median time to first infective exacerbation with inhaled liposomal ciprofloxacin when compared with placebo; however, this was not reflected in ORBIT-3.28 Similarly, in the RESPIRE 1 trial it was demonstrated that ciprofloxacin dry powder inhalation reduces the frequency of exacerbations in patients who have non–cystic fibrosis bronchiectasis with frequent exacerbations and pathogens present in sputum, compared with placebo.29
Conclusion
Bronchiectasis is a common chronic lung disease that is prevalent in the Australian community, especially in the Australian Aboriginal community. It significantly affects quality of life and increases healthcare costs; however, it is poorly reported and documented. Bronchiectasis is secondary to various underlying causes, and management is aimed at investigating and managing the primary causes with a focus on sputum clearance, reduction in exacerbations and improvement of quality of life.