Rural general practitioners (GPs) are largely responsible for delivering primary and secondary care to rural populations in Australia. This population is characterised by low population density, with large geographical distances separating the population centres. Rural generalists (RGs) in Australia have responded to these needs by expanding their professional capacities to incorporate advanced specialty skills such as obstetrics, anaesthesia, surgery and gastrointestinal endoscopy. Although the exact numbers are unknown, GP endoscopists form the minority of endoscopists, with the majority being surgeons and gastroenterologists. There is limited literature investigating the performance of GP endoscopists.
GP endoscopists are trained to perform colonoscopies through the standardised Gastroenterological Society of Australia (GESA) certification program, with recertification after three years, as for all endoscopists in Australia. Compared to other states, Queensland has a relatively high number of rural generalists, as training is facilitated through the Queensland Rural Generalist Pathway. Despite this, many rural hospitals have no GP endoscopists. Darling Downs Health is one of the major rural health precincts in Queensland. It covers a geographic region of 90,000 square kilometres, with a population that was expected to reach 295,000 by 2021–22.1 Even in this region, there are only four GP endoscopists in the seven rural hospitals with theatre services as of 2018–21.
Colorectal cancer (CRC) is the second most common cause of cancer-related death in Australia.2 In 2006, the Australian Government introduced the National Bowel Cancer Screening Program (NBCSP), which provides a cost-free faecal immunochemical test (FIT) every two years for individuals aged 50–74 years.3 Patients with positive FIT results are recommended for colonoscopy, a process that is usually facilitated by the GP.4
Colonoscopy is used as a diagnostic, therapeutic and preventive tool for CRC. With the nationwide rollout of the NBCSP, the demand for colonoscopy services in Australia is rising. The NBCSP participation rate is lowest in very remote areas.3,5 The concern is that the demand may not be met in rural communities, where access to healthcare is limited compared to urban centres, particularly with regards to specialist services.6 Hence, rural GPs are encouraged to upskill in performing colonoscopy.
In line with international standards, GESA sets quality standards for endoscopists practising in Australia by governing training, recertification and performance review.7
The aim of this study was to investigate the colonoscopy performance of three Australian GP endoscopists in rural Queensland. This involved comparison against the current Australian quality indicator (QI) standards.
Methods
Study design
A cross-sectional study was performed, investigating the colonoscopies undertaken between January 2018 and February 2021 by three rural GP endoscopists in three rural hospitals: Warwick, Stanthorpe and Longreach in Queensland, Australia.
Warwick Hospital is a rural hospital located 70 minutes from the nearest referral centre and over two hours’ drive from Brisbane. Stanthorpe Hospital is located three hours from the nearest referral centre. Longreach Hospital is in outback Queensland, over two hours by air from Brisbane. The Modified Monash Model (MMM) is a classification system developed by the Australian Department of Health to stratify rurality from Modified Monash (MM) 1 (metropolitan) to MM 7 (very remote).8 Warwick and Stanthorpe are classified as medium rural towns (MM 4) and Longreach as a very remote community (MM 7). The patients’ recorded primary addresses were classified using the MMM database.
Almost all colonoscopies were performed under sedation provided by GP anaesthetists. The exclusion criteria included cases more appropriately performed outside rural hospitals, such as known colonic lesions requiring advanced endoscopic resection or patients with significant anaesthetic risks due to medical comorbidities.
Data extraction
Data was extracted from the Operating Room Management Information System (ORMIS) using Crystal Reports. Inclusion criteria included colonoscopies performed by rural GP endoscopists between January 2018 and February 2021. The exclusion criteria included data on flexible sigmoidoscopies and colonoscopies performed by non-rural GP endoscopists or proceduralists who had not performed at least 150 colonoscopies within a three-year period.
The extracted data included patient demographical information including age, sex and primary residential address; procedural reports; histopathology; and clinical encounters. The Viewer is a portal that integrates patient data from public Queensland Health systems. This served as a central source of data for emergency department (ED) presentations, post-procedure hospital admissions, and discharge summaries in all Queensland public hospitals.
ORMIS provided the procedure date, patient medical record number, age, sex and American Society of Anesthesiologists (ASA) physical status. Colonoscopy reports are created in the Provation MD software system containing the colonoscopy indications, documentation, photographs of the colonoscopy endpoint, bowel preparation quality, numbers of polyps identified/resected, other relevant findings such as diverticulosis and CRC, total colonoscopy time and withdrawal time. Colonoscopy completion is defined as reaching the caecum proximal to the ileocaecal valve or terminal ileum in intact colons. Otherwise, incompletion is recorded along with the reasons. The withdrawal time refers to the time taken to inspect the colon, from the time of reaching the caecum to colonoscopy completion. Cancer Council Australia recommends the Boston bowel preparation scale (BBPS) to evaluate bowel preparation quality.9 In our study, unsatisfactory bowel preparation was defined as inadequate, poor bowel preparation or BBPS <6, requiring repeat colonoscopy.
Calculations and data analysis
Microsoft Excel and SPSS version 26 were used to store and analyse data. The primary QIs include caecal intubation rate (CIR), adenoma detection rate (ADR), serrated polyp detection rate (SDR), clinically significant SDR (CSSDR) and mean withdrawal time. CIR is the proportion of colonoscopy completion, defined by reaching the caecum. ADR was found by calculating the proportion of adenomas confirmed by pathologists in patients who were aged ≥50 years, with intact colons and without inflammatory bowel disease as an indication for their colonoscopy. Adenocarcinomas were not included in ADR. The World Health Organization classifies a serrated lesion as a sessile serrated lesion (SSL), previously known as a sessile serrated adenoma/polyp (SSA/P), hyperplastic polyp (HP) and traditional serrated adenoma (TSA). SDR was calculated through the proportion of any SSL, HP and TSA that were proximal to the sigmoid colon (not including the sigmoid colon) in patients who were aged ≥50 years with intact colons. CSSDR was calculated as the proportion of any SSL, TSA and >1 cm HP anywhere in the colon or >5 mm HP proximal to the sigmoid colon among patients aged ≥50 years with intact colons. We also studied polyp detection rates in different groups separated by age, gender and faecal occult blood test (FOBT) status.
Univariate comparisons in ADR, SDR and SDDR between the categories were conducted using chi-square analysis in SPSS.
Ethics approval
Ethical approval was from the Darling Downs Health Human Research Ethical Committee and Research Governance Committee of the Central West Hospital and Health Service (LNR/2020/QTDD/66128).
Results
Between January 2018 and February 2021, 1674 colonoscopies were performed by the three rural generalists. The patient age range was 16–93 years, and there were more males than females. Most patients lived in medium rural (MM 4) or more remote areas. More than 90% total patients were ASA 1 (normal healthy) or ASA 2 (mild systemic disease). Patient characteristics and colonoscopy indications are presented in Table 1.
| Table 1. Patient demographics, clinical baseline characteristics and indications of colonoscopy (n = 1674) |
| Patient characteristics |
n |
% |
| Sex |
|
|
| Male |
868 |
51.8 |
| Female |
806 |
48.1 |
| Age (years) |
| <40 |
160 |
9.6 |
| 41–50 |
213 |
12.7 |
| 51–60 |
437 |
26.1 |
| 61–70 |
502 |
30.0 |
| 71–80 |
306 |
18.3 |
| ≥81 |
56 |
3.3 |
| Modified Monash Model |
| 1 |
4 |
0.2 |
| 2 |
9 |
0.5 |
| 3 |
1 |
0.06 |
| 4 |
928 |
55.4 |
| 5 |
528 |
31.5 |
| 6 |
2 |
0.1 |
| 7 |
198 |
11.8 |
| Missing data |
4 |
0.2 |
| ASA physical status |
| 1 |
169 |
10.1 |
| 2 |
1,360 |
81.2 |
| 3 |
139 |
8.3 |
| Missing data |
6 |
0.4 |
| Colonoscopy indications* |
| Positive FOBT (non-NBCSP and NBCSP) |
625 |
29.8 |
| Anaemia |
104 |
5.0 |
| Haematochezia |
355 |
17.0 |
| Diverticulosis |
38 |
1.8 |
| Abdominal pain |
152 |
7.2 |
| Change of bowel habits |
283 |
13.5 |
| Weight loss |
8 |
0.4 |
| Polyp surveillance |
316 |
15.1 |
| History of CRC |
41 |
2.0 |
| Family history of CRC |
146 |
7.0 |
| Genetic predisposition |
13 |
0.6 |
| Inflammatory bowel disease |
13 |
0.6 |
*Some colonoscopies have multiple indications.
ASA, American Society of Anesthesiologists; CRC, colorectal cancer; FOBT, faecal occult blood test; NBCSP, National Bowel Cancer Screening Program |
Table 2 summarises the QIs. There was a statistically significant difference in ADR between males (55.6%) and females (42.2%), P <0.001. There was a significantly higher SDR among males (18.1%) when compared with females (13.5%), P = 0.02. Similarly, there was a significantly higher CSSDR in males (16.4%) than females (11.4%), P = 0.01.
| Table 2. Colonoscopy characteristics, primary QIs, QIs categorised by FOBT status/age and poor bowel preparation cases |
| Colonoscopy characteristics |
n |
|
| Total colonoscopies |
1,674 |
|
| Intact colons |
1,623 |
|
| Intact colons excluding IBD |
1,610 |
|
Intact colon (patient aged
≥50 years) |
1,278 |
|
| Male |
700 |
|
| Female |
578 |
|
| QIs |
% |
| Completion rate |
1,587 |
97.8 |
| ADR (M+F)* |
633 |
49.5 |
| M |
389 |
55.6 |
| F |
244 |
42.2 |
| SDR (M+F)* |
205 |
16.0 |
| M |
127 |
18.1 |
| F |
78 |
13.5 |
| CSSDR (M+F)* |
181 |
14.2 |
| M |
115 |
16.4 |
| F |
66 |
11.4 |
| Mean withdrawal time (min) |
15.9 |
|
| Positive FOBT of all ages |
625 |
|
| ADR |
335 |
53.6 |
| SDR |
96 |
15.4 |
| Positive FOBT of patients aged ≥50 years |
565 |
|
| ADR |
319 |
56.5 |
| SDR |
88 |
15.6 |
| Positive FOBT of patients aged <50 years |
60 |
|
| ADR |
16 |
26.7 |
| SDR |
8 |
13.3 |
| Patient aged ≥50 years, indications other than FOBT |
713 |
|
| ADR |
314 |
44.0 |
| SDR |
117 |
16.4 |
| Poor bowel preparation |
102 |
6.1 |
*ADR, SDR and CSSDR calculated over intact colon and no IBD over 50 years of age
ADR, adenoma detection rate; CSSDR, clinically significant serrated polyp detection rate; F, female; FOBT, faecal occult blood test; IBD, inflammatory bowel disease; M, male; QIs, quality indicators; SDR, serrated polyp detection rate |
Additionally, ADR and SDR were examined categorised by FOBT status and age. Chi-square analysis revealed that the ADR among FOBT-positive patients aged ≥50 years (56.5%) was significantly higher than those aged <50 years (26.7%), P <0.001. However, no statistically significant difference in SDR was found between FOBT-positive patients aged ≥50 years and those aged <50 years (P = 0.65).
Over each year, poor bowel preparation rate has consistently met the recommended threshold of <15% (Figure 1).10 Table 3 displays the reasons for incompletion of colonoscopy.
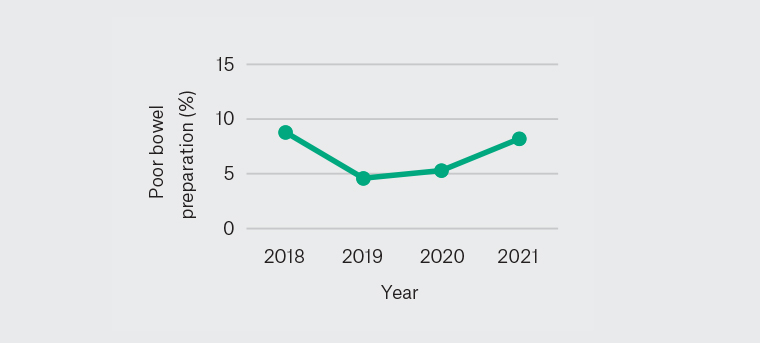
Figure 1. Poor bowel preparation rate 2018–21
| Table 3. Reasons for incompletion of colonoscopy |
| |
n |
% |
| Poor bowel preparation |
16 |
44.4 |
| Technical (tortuous, sharp angle) |
11 |
30.6 |
| Colonic stenosis/obstruction |
5 |
13.9 |
| Anaesthetic/patient medical conditions |
3 |
8.3 |
| Others |
1 |
2.8 |
| Total |
36 |
100 |
No statistical difference was found in QIs and poor bowel preparation rate between each year (Figures 1–5).
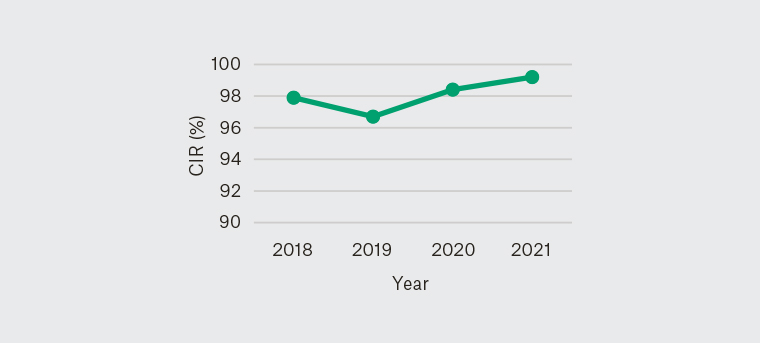
Figure 2. Yearly caecal intubation rate 2018–21
CIR, caecal intubation rate
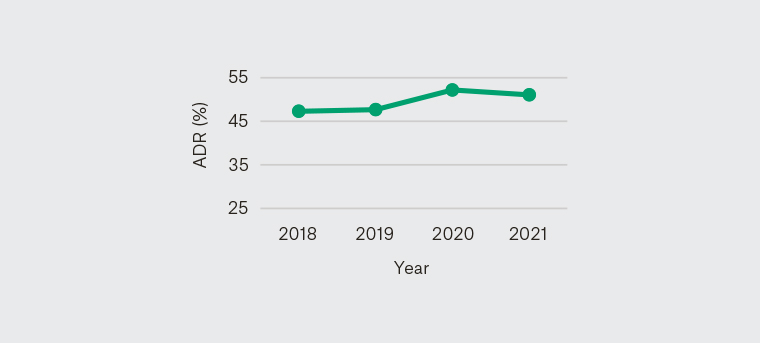
Figure 3. Yearly adenoma detection rate 2018–21
ADR, adenoma detection rate
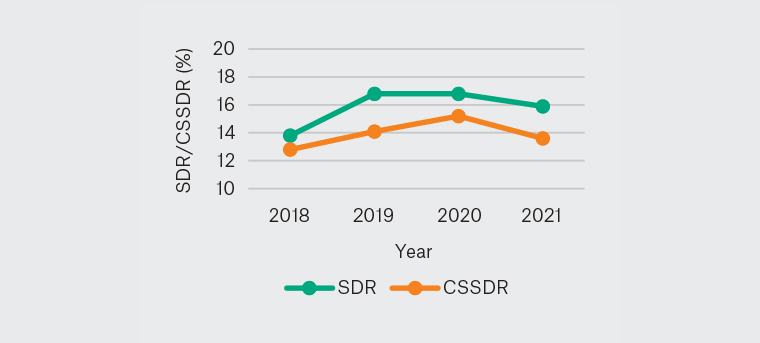
Figure 4. Yearly SDR and CSSDR 2018–21
CSSDR, clinically significant serrated polyp detection rate; SDR, serrated polyp detection rate
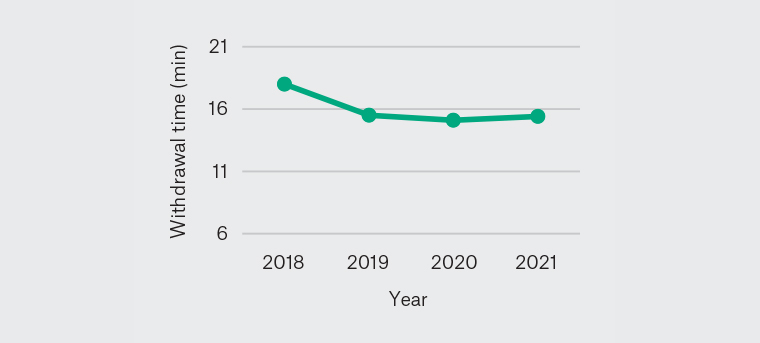
Figure 5. Yearly mean withdrawal time 2018–21
Table 4 shows hospital representations (0.9%) and major complications within the 30-day post-procedure period (0.06%).
| Table 4. Reasons for presentation to emergency department in 30-day period post colonoscopy |
| Days* |
Reasons |
Outcome/management |
| 0 |
Nausea/dizziness |
Symptom Mx (ED presentation only) |
| 1 |
Abdominal pain/haematochezia |
Ischaemic colitis – Symptom Mx (two‑day admission) |
| 1 |
Abdominal pain |
Symptom Mx (two-day admission) |
| 1 |
Abdominal pain/dizziness |
Symptom Mx (ED presentation only) |
| 2 |
Haematochezia (post-banding) |
Symptom Mx (ED presentation only) |
| 2 |
Headache/dehydration |
Symptom Mx (ED presentation only) |
| 2 |
Abdominal pain |
Perforation: antibiotic/analgesia (three‑day admission) |
| 3 |
Abdominal pain/PV bleeding |
Other diagnosis (menorrhagia) |
| 4 |
Vertigo |
Symptom Mx (two-day admission) |
| 4 |
Rectal pain/diarrhoea |
Symptom Mx (ED presentation only) |
| 5 |
Abdominal pain |
Symptom Mx (ED presentation only) |
| 7 |
Nausea/dizziness |
Symptom Mx (ED presentation only) |
| 9 |
Fever/nausea |
Other diagnosis (fever of unknown origin) |
| 15 |
Collapse |
TIA |
| 27 |
Abdominal pain |
Other diagnosis (renal colic) |
*Number of days post colonoscopy
ED, emergency department; Mx, management; PV, per vaginal; TIA, transient ischaemic attack |
Discussion
Based on QIs regarding performance of the three rural Queensland GPs, our findings suggest that GP endoscopists can perform colonoscopies to a high standard.
Indications
Table 1 demonstrates that the highest proportion of colonoscopies performed in the study were indicated for positive FOBT (29.8%), followed by haematochezia (17.0%) and polyp surveillance (15.1%). It is to be noted that some colonoscopies have multiple indications, and the validity of an indication is determined on a case-by-case basis. For instance, the appropriate time frame for interval colonoscopies for polyp surveillance would vary based on factors including polyp number, size and degree of dysplasia, villosity, serrated lesions or previous polyp resection. Endoscopists must also ensure that the clinical indication fulfills the national eligibility requirement for the Medicare Benefits Schedule rebate.
Caecal intubation rate
Consequences of incomplete colonoscopies include missed lesions and failure to prevent CRC.11 It is accepted that not all colonoscopies can be completed to the caecum due to reasons such as poor bowel preparation, tortuous colon, strictures or malignant obstruction.10,12
A UK national audit demonstrated an unadjusted CIR of 92.3%; following adjustment for impassable strictures and poor bowel preparation, the CIR was 95.8%.13 GESA sets a target CIR of 90% in training and 95% for three-yearly recertification.7,14 To prove caecal intubation, endoscopists are required to record clear photoidentification of the ileum or caecum showing the appendiceal orifice and ileocecal valve.10 In this study, the overall completion rate was 97.8%, exceeding this benchmark. Table 3 displays the reasons for incompletion, which are consistent with the reasons acknowledged in the literature. It is to be noted that CIR, excluding non-proceduralist dependent reasons, was 99.3%.
Adenoma detection rate
ADR is one of the key colonoscopy QIs.15 GESA has established that an ADR of 25% is consistent with international standards.7 The overall ADR in individuals aged ≥50 years with intact colons in this study was 49.5%. Recommended ADR is influenced by age and sex; in this study, the ADR among male patients (55.6%) and female patients (42.2%) surpassed the gender-specific targets of 30% and 20% respectively.10
In patients with positive FOBT, the overall ADR was 53.6%, being significantly higher among those aged ≥50 years (56.5%) than those aged <50 years (26.7%). This suggests that positive FOBT results in younger populations are likely due to reasons other than adenomas. Moreover, for those aged ≥50 years, ADR in FOBT-positive patients (56.5%) is higher than primary ADR (49.5%), suggesting the sensitivity of FOBT to adenomas.
SDR and CSSDR
SDR and CSSDR are recently established quality indicators that represent the detection of serrated lesions.16 The sessile neoplastic pathway describes the progression of serrated polyps to CRC.17,18 It is now established that serrated lesions are premalignant lesions, accounting for 5–30% of CRCs.19,20 Of note, interval CRCs are more commonly to be found from serrated lesions in the proximal colon.17,20
The detection of serrated lesions, particularly SSLs, is difficult due to their flat morphology and indistinct borders.17 GESA set an initial benchmark of minimum 4%.
The rural GP endoscopists in this study achieved an SDR of 16.0% and a CSSDR of 14.2%. It was found that SDR and CSSDR were significantly higher among male patients than female patients. Unlike ADR, however, the SDR did not reveal any significant differences between the age groups among FOBT-positive patients. This indicates that the prevalence of serrated lesions in young (<50 years) patients is relatively high, echoing findings of a previous study.21
It is noted that SDR was high (16.4%) in patients who had colonoscopies for indications other than FOBT (Table 2). Previous studies have demonstrated poor sensitivity of FOBT to serrated lesions.22 This reinforces the importance of high-quality colonoscopies. In cases of missed serrated lesions, subsequent FOBT screening may not assist in detecting serrated lesions. There is potential for future studies to investigate the association between SDR and specific indications other than FOBT to improve serrated lesion detection.
Colonoscopy withdrawal time
Increased colonoscopy withdrawal time is associated with increased detection of colonic lesions because it enables more time and opportunity to find polyps.23,24 It is to be noted that endoscopists may inspect the colon, detect polyps and perform polypectomies during colonoscopy insertion, suggesting that the withdrawal time may not represent the entire surveillance time. Moreover, the withdrawal time may vary depending on colon conditions such as colonic length, spasm and quality of bowel preparation. While GESA has not set a benchmark withdrawal time for the recognition of training and recertification criteria, the GESA logbook record recognises the importance of withdrawal time. The NBCSP recommends withdrawal times of at least six minutes as it is associated with higher polyp detection rates consistent with international recommendations.10,12 Previous studies have demonstrated statistically significant differences in ADR between groups with withdrawal times <6 minutes and ≥6 minutes.25,26 The mean withdrawal time in this study was 15.9 minutes and remained relatively consistent throughout the study period.
Unexpected outcomes: Complication or hospital re-presentation
Colonoscopies are associated with risks such as bleeding, perforation, infection and anaesthetic adverse events. In this study, the procedures were safe with a low complication rate. While most did not result in clinically significant medical consequences, the culture of seeking early medical attention may enable unforeseen complications to be detected and managed within an appropriate time frame. Hospital admissions for monitoring reflected the safety net that is required for patients who reside rurally and may experience barriers with accessing healthcare in a timely manner.
Reported perforation rates vary between studies.10,12,27 A systematic review reported perforation rates as low as 0.007% for screening colonoscopies.28 Based on large population studies, the American Society for Gastrointestinal Endoscopy sets the performance target as <1:1000 perforation in screening.10 This study identified one perforation in a patient with known diverticulosis, where the colonoscopy was indicated to investigate multiple abdominal symptoms. The colonoscopy was completed without difficulty and no polypectomies were performed; procedural findings included diverticulosis in the sigmoid and ascending colons. The patient re-presented two days later with abdominal pain. An abdominal computed tomography scan showed small air bubbles and inflammation in the ascending colon, consistent with perforation. The patient was transferred to a regional referral hospital and required three days of hospital admission with antibiotic treatment prior to discharge. The patient did not require surgery.
The incidence of post-polypectomy bleeding is <1%.10,29 Delayed bleeding is more likely to occur when electrocautery is used for polypectomies.10 In our study there was no reported case of clinically significant bleeding. This could be due to the selection of procedures to be safely performed in rural/remote hospitals, such as in the selection of patients with low-risk pre-anaesthesia medical conditions, selection of polypectomy size, avoidance of advanced colonoscopy procedures and use of haemostatic clips at lower thresholds.
This study reports the outcomes of colonoscopies performed by three GP endoscopists in rural Queensland. The GP endoscopists in our study were trained by a specialist surgeon, gastroenterologist and/or accredited GP endoscopist in public hospitals and/or endoscopy centre through the GESA certification program and hold colonoscopy recertification assuring continual competency. To improve the generalisability of findings and examine factors in different centres that might influence colonoscopy performance, such as endoscopist training background and pre- and post-colonoscopy practices, future studies should consider collating data from large numbers of rural GP endoscopists at different centres across Australia. Such studies might suggest a framework for increasing rural GP endoscopist training and creating a standardised, qualified workforce.
Conclusion
The present multicentre study demonstrated that the three studied GP endoscopists delivered safe, high-quality colonoscopy services for their respective rural communities in Queensland. This study supports the prospect of expanding rural GP endoscopy services to meet increasing demands in a safe, effective manner.