Case
A man aged 36 years with a history of hypertension presented to discuss his recent blood test results. His past medical history included hypertension for 1.5 years that was treated with olmesartan and amlodipine, and dyslipidaemia that was treated with atorvastatin. He was adherent to his antihypertensive therapy and had normal electrolytes until the time of presentation.
Blood test results showed a serum potassium of 3 mmol/L (reference range 3.6–5.4 mmol/L), which was persistent on subsequent tests. Other electrolytes, glucose levels and renal function were normal.
Question 1
What are the causes of hypertension and hypokalaemia?
Question 2
How would you definitively diagnose the patient?
Answer 1
Causes of hypertension and hypokalaemia are detailed in Table 1.
| Table 1. Causes of hypertension and hypokalaemia10 |
| Cause |
Comments |
| Primary aldosteronism |
Due to nonsuppressible hypersecretion of aldosterone. PRC is typically low and PAC is elevated |
| Secondary hyperaldosteronism (diuretic use, renovascular hypertension, renin-secreting tumours) |
Hypersecretion of renin leads to increased angiotensin II and then increased aldosterone secretion |
| Cushing’s syndrome |
Due to overproduction of deoxycorticosterone, corticosterone and cortisol, which act as mineralocorticoids and cause a suppressed PRC and a low PAC. Diagnosis is suspected from the classic cushingoid appearance |
| Liddle’s syndrome |
Due to a gain-of-function mutation in the collecting tubule sodium channel, which causes an increase in sodium reabsorption and, in most cases, potassium secretion. Both PRC and PAC are low |
| Chronic liquorice root ingestion |
Liquorice contains a steroid that inhibits the enzyme that converts cortisol to cortisone. Cortisol binds as avidly as aldosterone to the mineralocorticoid receptor. Both PRC and PAC are low |
| Congenital adrenal hyperplasia (11β-hydroxylase deficiency) |
Hypersecretion of the mineralocorticoid deoxycorticosterone causes a suppressed PRC and a low PAC |
| PAC, plasma aldosterone concentration; PRC, plasma renin concentration |
Answer 2
After excluding diuretic and herbal medicine use, the next step in a patient with hypertension and hypokalaemia is to screen for primary aldosteronism (PA).
The prevalence of PA is higher in a number of patient groups (Box 1), including patients with hypertension and hypokalaemia.
| Box 1. High-risk groups for primary aldosteronism1 |
- Patients with sustained BP >150/100 mmHg on all three measurements obtained on different days
- Patients with hypertension (BP >140/90 mmHg) resistant to three conventional antihypertensive agents, including a diuretic
- Patients with controlled BP (<140/90 mmHg) on four or more antihypertensive agents
- Patients with hypertension and spontaneous or diuretic-induced hypokalaemia
- Patients with hypertension and adrenal incidentaloma
- Patients with hypertension and sleep apnoea
- Patients with hypertension and a family history of early-onset hypertension or cerebrovascular accident at a young age (<40 years)
- All patients with hypertension who have a first-degree relative with primary aldosteronism
|
| BP, blood pressure |
However, only a minority of patients with PA (9–37%) have hypokalaemia.1
An aldosterone-to-renin ratio (ARR) is recommended as a screening test in high-risk groups to detect possible cases of PA.1
A number of antihypertensive medications can interfere with the ARR (Table 2).
| Table 2. Effects of antihypertensive drugs on ARR2 |
| Drug class |
Effect on ARR |
Minimum withdrawal period before ARR testing |
| Diuretics (MRAs, loop diuretics, thiazides) |
False negative |
4 weeks |
| ACEIs and ARBs |
False negative |
2 weeks |
| Beta-blockers |
False positive |
2 weeks |
| Dihydropyridine CCBs |
False negative |
2 weeks |
| ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; ARR, aldosterone-to-renin ratio; CCBs, calcium channel blockers; MRAs, mineralocorticoid receptor antagonists |
Most antihypertensive medications can be continued, but the ARR result should be interpreted with consideration of the effect of the potential interfering medication. For example, in a patient who takes an angiotensin-converting enzyme inhibitor that decreases the ARR, an elevated ARR strongly suggests PA, whereas a normal ARR in a patient who takes a β-blocker (which increases the ARR) makes PA unlikely.
If interfering antihypertensive medications are withdrawn before ARR, alternative antihypertensive medications with minimal effects on the ARR (eg verapamil slow release, hydralazine or an α-adrenergic blocker such as prazosin) can be commenced, when necessary.
It is important to consider potential risks of adjusting antihypertensive treatment (eg hypertensive crisis, heart failure, atrial fibrillation) prior to ARR measurement.
Liberalisation of sodium intake and correction of hypokalaemia are recommended prior to ARR measurement.1–3
Patients with an elevated ARR are recommended to undergo a confirmatory test such as a saline infusion test or oral sodium loading test to definitively confirm or exclude the diagnosis of PA.1
Confirmatory tests can be omitted in patients with a low plasma renin concentration (PRC) and a plasma aldosterone concentration (PAC) >830 pmol/L irrespective of serum potassium level, or in patients with hypokalaemia who have a low PRC and a PAC between 550 pmol/L and 830 pmol/L.4 This is because in this clinical setting there is no other diagnosis except PA to explain these findings.3
Case continued
The patient’s physical examination was unremarkable. He was not on any diuretics or herbal medicines, so the next step was to screen for PA.
Question 3
What is PA?
Question 4
What are the causes and clinical features of PA?
Question 5
What further investigations are indicated in a patient with confirmed diagnosis of PA?
Answer 3
PA, also called Conn’s syndrome, is a group of disorders characterised by excessive production of aldosterone that is relatively autonomous of the renin-angiotensin system and non-suppressible by sodium loading.1,5
Answer 4
The most common causes of PA are adrenal adenomas and bilateral adrenal hyperplasia. It is less commonly caused by unilateral adrenal hyperplasia, adrenal carcinomas or familial hyperaldosteronism.3
Excessive aldosterone production causes sodium retention, hypertension, cardiovascular damage (eg left ventricular dysfunction, myocardial infarction, arrhythmias, stroke), renal dysfunction (eg glomerular hyperfiltration, increased urinary albumin excretion) and increased urinary potassium losses that, if prolonged and severe, may result in hypokalaemia. PA is also associated with impaired physical and mental quality of life. Patients may experience symptoms such as decreased energy, poor sleep quality, anxiety, depression, demoralisation and nervousness.1,6,7
Answer 5
All patients with confirmed diagnosis of PA are recommended to undergo adrenal computed tomography (CT) to exclude adrenocortical carcinoma and help determine subtype (unilateral adenoma or bilateral hyperplasia).
For patients who desire surgical treatment and have no comorbidities that could increase surgical risk, an experienced radiologist should perform adrenal venous sampling (AVS), which is the gold standard test to distinguish between unilateral adenoma and bilateral adrenal hyperplasia.1
In younger patients (aged <40 years) with spontaneous hypokalaemia, marked aldosterone excess and a unilateral adrenal adenoma on a CT scan, AVS may not be needed prior to unilateral adrenalectomy.8 This is because the possibility of having a non-functioning adrenal adenoma that could be confused with an aldosterone-producing adenoma is low in these patients.3
Case continued
The patient was commenced on oral potassium tablets in consultation with the endocrinology team at a tertiary hospital. His electrolytes were monitored regularly.
To prepare the patient for ARR measurement, his antihypertensives were ceased and he was commenced on verapamil sustained release. His blood pressure was monitored during the withdrawal period. Two weeks later his ARR was 88.3 (reference range <70), PRC was 12 mU/L (reference range 4.4–46.1 mU/L) and PAC was 1060 pmol/L (reference range 71–1235 pmol/L). Both PRC and PAC were measured in an upright position.
To confirm the diagnosis of PA, a saline infusion test was performed that revealed a post-infusion PAC of 366 pmol/L (levels >280 pmol/L are suggestive of very probable PA).1
The patient also underwent an adrenal CT scan, which revealed a 15 mm × 16 mm × 15 mm left-sided adrenal adenoma (Figure 1).
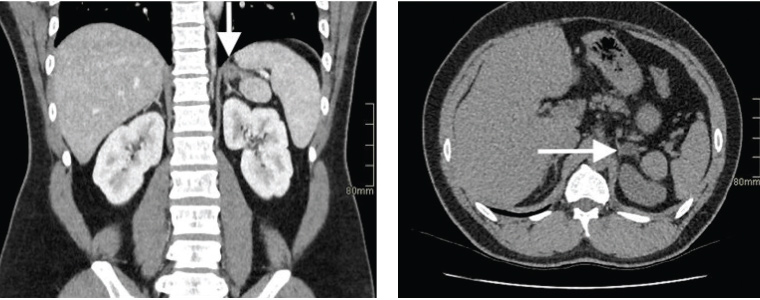
Figure 1. Adrenal computed tomography demonstrating a left adrenal adenoma (arrows)
Question 6
What is the epidemiology of PA?
Question 7
How is PA managed?
Answer 6
PA was previously thought to account for <1% of patients with hypertension. Introduction of ARR as a screening test has resulted in a higher prevalence of this disease. PA is now the most common form of hypertension that can be cured or specifically treated.5 It is estimated to account for 5–13% of patients with hypertension.2
Answer 7
Unilateral laparoscopic adrenalectomy is the preferred treatment for patients with documented unilateral PA.1
Hypertension should be controlled and hypokalaemia should be corrected with potassium supplementation or a mineralocorticoid receptor antagonist (MRA) such as spironolactone or eplerenone during the pre-operative period.9
In patients with unilateral disease who are unwilling or unable to undergo surgery, and patients with bilateral disease, medical treatment with an MRA is recommended. Spironolactone is suggested as the drug of choice. The starting dose for spironolactone is 12.5–25 mg/day in a single dose, which can be increased very gradually, if necessary, to a maximum dose of 100 mg/day. Eplerenone (not listed on the Pharmaceutical Benefits Scheme for this indication) is given as an alternative agent if the patient develops endocrine side effects from spironolactone. The starting dose for eplerenone is 25 mg twice daily.1
Studies show either surgical or pharmacological treatment of PA can improve not only the cardiovascular/renal morbidity caused by aldosterone excess, but also the quality of life of patients.1,6,7
Case continued
The patient was referred to an endocrine surgeon. His hypokalaemia and hypertension were managed with oral potassium supplements and antihypertensives in the pre-operative period.
He underwent a laparoscopic left subtotal adrenalectomy a few months later. Histopathology confirmed adrenal cortical adenoma. Antihypertensive agents and potassium tablets were ceased upon normalisation of his blood pressure and potassium levels following the operation. He also reported improved quality of life at his follow-up consultation one month after surgery.
Key points
- PA is the most common curable or specifically treatable form of hypertension.
- PA has adverse cardiovascular and renal consequences.
- Early detection and treatment of PA minimises target organ damage caused by this condition.