Methadone and buprenorphine are evidence-based effective medication-assisted treatments for opioid dependence (MATOD) that are best used with psychosocial support.1 The national treatment guidelines consider that general practice provides optimal access to MATOD, with support from specialist services.1 Sublingual buprenorphine products have been licensed in Australia since 2000. More recently, two injectable depot formulations of buprenorphine have been licensed and publicly funded for MATOD within a framework of medical, social and psychological treatment. Generally, treatment with the monthly depot used in this study is commenced following induction with sublingual buprenorphine.1
Retention in MATOD is key, given that discontinuation and relapse significantly increase all cause and overdose mortality risk; longer retention in treatment (>12 months) reduces mortality risk.2 However, only one recent study reporting retention rates in Australia was identified, which reported a one-year retention of 48% with sublingual buprenorphine and 59% with methadone in New South Wales.3 In a phase 3 US study of the depot buprenorphine product in the current study, 62–64% of patients completed 24 weeks of double-blind treatment (versus 34% of placebo recipients).4
In this study, we report retention in treatment during early experience with a monthly buprenorphine depot at a general practice clinic in Melbourne, Australia. Overall retention was calculated; that is, all patients who remained on treatment at the end of the study, allowing flexibility in the number of days between doses. Occasional delays are not expected to have clinically significant effects on treatment if steady state has been achieved (ie a consistent plasma concentration of buprenorphine),5 and withdrawal effects are unlikely within 6–8 weeks after the last monthly depot injection.6 The product registration allows up to 14 days’ delay between scheduled treatments (ie 42 days between treatments),5 and Victoria state guidelines permit one dose to be missed (ie 56 days between injections).7 Therefore retention was also calculated using more stringent definitions of discontinuation; that is, a delay of two or four weeks beyond the scheduled 28-day interval between injections was classified as discontinuation.
This study describes the patient demographics and estimates the retention rate in patients receiving monthly depot buprenorphine at a general practice clinic over an approximate 12-month period.
Methods
Study design
This single-arm retrospective cohort study involved patients who are opioid dependent at a mixed-billing, Medicare Benefits Schedule (MBS)-funded general practice clinic in a metropolitan-suburban setting in Melbourne. Patients were managed by two general practice MATOD prescribers with >20 years’ experience and a recently accredited prescriber. (Note that the legislation and regulations determining how many patients a general practitioner can treat vary among Australian states and territories.) Opioid-dependent patients attending the general practice and all patients already receiving MATOD at the general practice clinic were offered treatment with the monthly buprenorphine depot. Patients underwent comprehensive clinical review, mostly by the first author (EA), who manages >900 opioid-dependent patients. Patients on methadone were transferred as outpatients to sublingual buprenorphine using microdosing with buprenorphine sublingual film (the Bernese method).8 Demographic data, opioid use history, sublingual dose of buprenorphine prior to transfer to depot buprenorphine, clinic visit dates and depot dose administered were obtained from clinical records. Data were collected between 11 December 2019 and 30 November 2020. Low-risk ethics approval was obtained from Bellberry Pty Ltd to analyse specific aspects of medical records retrospectively (no.: 2020-10-89).
Depot buprenorphine was administered by subcutaneous injection, as per the product information.5 While patients already on sublingual buprenorphine can be switched immediately to the depot formulation, patients were asked to take their current sublingual dose 10–15 minutes before the first injection because of the potential risk of precipitated withdrawal.
Standard practice at this general practice clinic is to administer local anaesthetic (xylocaine 2% without adrenaline) before each of the first two 300 mg subcutaneous injections. After these first two doses, patients either continued on the same dose for maintenance or decreased to 100 mg (ie the recommended maintenance dose), depending on their response to the first two 300 mg injections. Maintenance injections were administered by the practice nurse, and patients were offered ice packs or local anaesthetic before the injection. The depot injections were delivered without cost to the practice and were stored in a Schedule 8–compliant fridge. The MBS covered patient costs.
Appointments were booked for patients to return for their next injection after 28 days. Attempts were made to contact patients who did not rebook appointments. Patients were followed for up to 168 days (ie six depot buprenorphine injections planned to be given at 28-day intervals).
Patients were offered psychosocial support as required, which could have included a care coordinator to identify concurrent issues requiring referral to allied health support, mental healthcare plans, inhouse psychology or counsellor meetings. The aim of this psychosocial support was to help identify triggers for relapse or continued opioid use, or as adjuvant treatment for mental or physical illnesses.
Calculation of retention rates
The duration of retention in treatment was determined as the date of the last dose minus the date of the first dose + 29 days. The overall retention rate was defined as the percentage of patients still on treatment at day 168, independent of the number of days between doses. The retention rate was also calculated using ‘discontinuation’, defined as a delay of two or four weeks beyond the scheduled 28-day interval between any two depot injections (2/4WTD). Therefore, patients who had intervals between any two depot injections of >42 days (2WTD) and >56 days (4WTD) were considered to have discontinued treatment.
Data analysis
Data were managed and analysed using SAS (version 9.4). The LIFETEST procedure was used to generate Kaplan–Meier (KM) estimates for retention. KM analysis is an established statistical method used to determine approach for time-to-event analysis.9 In this case, the event of interest was unplanned discontinuation, and the time in the analysis was measured from the start of the depot buprenorphine treatment until unplanned discontinuation, planned transition or completion of a minimum course of treatment (six doses). KM analysis was the appropriate method for this analysis because it allows patients to enter the study at different time points, and it accounts for various lengths of follow up and different reasons for leaving the study. Patients who did not experience the event of interest (ie unplanned cessation of intended maintenance treatment) were censored if they remained on treatment for 168 days or were on treatment at the end of follow up (30 November 2020). Patients were also censored if they successfully completed a planned transition away from depot buprenorphine. Censoring is a way of distinguishing patients who did not have an event during follow up from those who did. It is commonly used when calculating the KM estimates. No statistical tests were performed.
Results
Patient characteristics
A total of 126 patients started treatment with depot buprenorphine between 11 December 2019 and 31 July 2020. Their mean age was 40.2 years (range: 20–65 years), and 32% were women. Nearly all (98%) had a history of heroin dependence before starting treatment. The mean dose of sublingual buprenorphine prior to stabilisation was 10.8 mg (range: 0.4–32 mg). Three patients (2%) were new to treatment and 123 (98%) were transferred from existing MATOD (18 methadone, 105 sublingual buprenorphine).
Retention rates
Ten patients achieved their goal of completing MATOD treatment and successfully ceased medication during the study period; one patient discontinued due to planned pregnancy. These patients were censored in the analysis, as they are not considered to have ‘failed’ maintenance treatment.
KM curves are shown in Figure 1 and retention rates are presented in Table 1. Median retention was reached for the 2WTD group (127 days), but not for the 4WTD group or for overall retention (ie KM estimates were >50% at the end of follow up). The median time between doses was 28 days for all definitions of retention and for patients treated with either 100 mg or 300 mg maintenance dose.
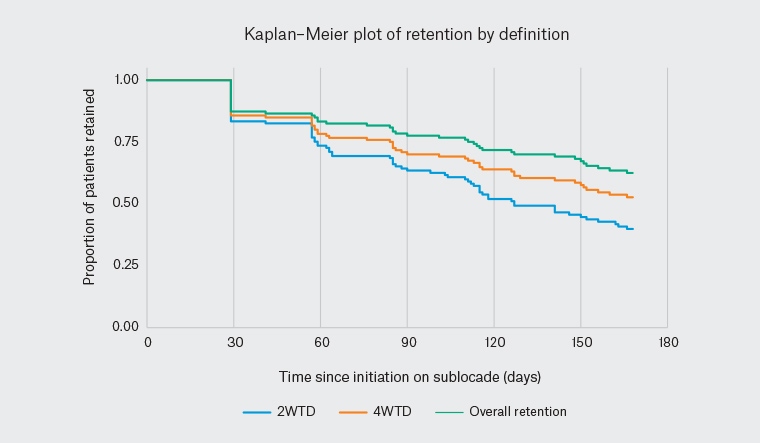
Figure 1. Kaplan–Meier estimates for retention in the study (n = 126)
| Table 1. Estimated retention at 168 days based on Kaplan–Meier analysis |
| Retention definition |
Median retention time |
Kaplan–Meier estimated retention rate |
| Two weeks’ treatment delay (ie patients were considered to have discontinued treatment if >42 days between any injections) |
127 days |
40% |
| Four weeks’ treatment delay (ie patients were considered to have discontinued treatment if >56 days between any injections) |
Not calculable |
53% |
| Overall retention (ie percentage of patients still on treatment at day 168) |
Not calculable |
62% |
Fifty-six patients (44%) discontinued treatment with the depot buprenorphine. The most common reasons were loss to follow up (38%, 21/56), planned discontinuation (20%, 11/56) and return to previous treatment (20%, 11/56). Additional reasons for discontinuation included permit transfer to another practice; patients without residency entitlement to MBS; and side effects, such as poor control of concurrent pain, insomnia or a feeling that the treatment was not ‘holding’ the patient as well as the previous medication. Twenty-nine per cent of patients (16/56) who discontinued treatment with the depot buprenorphine returned to the clinic to consult clinicians before discontinuation. Fourteen per cent (18/126) of patients dropped out after the first injection.
Eighty-six patients (68%) received at least four depot buprenorphine injections, and the majority of patients (87%) were stabilised on the standard 100 mg dose. None of the patients required supplemental sublingual buprenorphine during the study period.
Discussion
General practice has an important role in the treatment of opioid dependence. National treatment guidelines suggest that general practice delivery of MATOD ensures wide availability, helps reduce the stigma of addiction, assists patients to access general healthcare, and assists specialist drug and alcohol services to maintain capacity to assist patients new to treatment and those with complex presentations.1,10
The participants in this study broadly reflect the profile of patients with opioid dependency reported in the 2021 National Opioid Pharmacotherapy Statistics Annual Data (NOPSAD) collection on a snapshot day in June 2020 (n = 53,316): median age 44 years and two-thirds male (67%).11 However, heroin was the most commonly reported drug of dependence for 59% in NOPSAD,11 whereas 98% of our patient cohort were dependent on heroin before treatment.
The product registration allows up to 14 days’ delay to treatment (ie 2WTD),5 and Victoria state guidelines permit one dose to be missed (ie 4WTD).7 Occasional delays are not expected to have clinically significant effects on treatment, and models indicate that, once steady state has been achieved, plasma buprenorphine levels are maintained for 2–5 months after cessation of treatment with the monthly depot formulation used in this study.5 Therefore, the overall retention rate of 62% reported in this study is the most clinically relevant, as missing or delaying a monthly depot dose is unlikely to result in subtherapeutic dosing of buprenorphine.12 However, the median time between doses was 28 days, as per the prescribing information, indicating good concordance with the recommended dosing schedule. To encourage retention, the clinic books appointments at four-week intervals and recalls patients who have not booked follow-up appointments. However, patients often rebook for 5–6 weeks post-injection or miss an appointment and rebook when they feel the need. This study shows that occasional flexibility in the timing of depot injections is unlikely to compromise retention in treatment in contrast to sublingual buprenorphine or methadone, where missed doses could lead to relapse.13 However, it cannot be assumed that repeatedly extending the dosage interval beyond 28 days will not compromise effectiveness.
The overall retention rate with monthly depot buprenorphine reported in this general practice setting (62%) is similar to rates reported with sublingual buprenorphine and methadone across all settings at one year in New South Wales, Australia (48% and 59%, respectively).3 Furthermore, our rate in real-world general practice is close to the rate reported in the phase 3 study.4 Higher retention rates with depot buprenorphine have been reported during an early access scheme at three private referral clinics in Australia (67–93% retention at one year based on KM estimates).14 This could reflect different models of care and patient populations, and illustrates the balance between accessibility and quality of care referred to in the National Treatment Guidelines.1
Most of the study period occurred during the COVID-19 pandemic when lockdown measures restricted people’s movements.15,16 The clinic remained open during restrictions, but patients had to prebook appointments, and there were no walk-in clinics. Patients might have encountered difficulty travelling to the clinic, been reluctant to attend because of fear of exposure to COVID-19, or might have been isolating or quarantining. Consensus guidelines for MATOD during the pandemic included increased takeaway allowances,17 and national interim guidance recommended greater use of depot buprenorphine products and increased prescribing quotas and takeaway allowances (to one supervised dose and six takeaway doses each week), except for high-risk patients – a significant divergence from ‘routine care’ in Australia.17 The travel restrictions and availability of greater take-home allowances might have affected participants’ interest in monthly depot buprenorphine and retention on this treatment.
Monthly depot buprenorphine was offered to patients at the clinic who were motivated to stop MATOD but had been unable to reduce their daily oral or sublingual dose of buprenorphine or methadone despite repeated efforts to do so, and to new patients who struggled to attend the pharmacy daily. For some patients, daily medications are inconvenient, whereas monthly depot injections could provide greater flexibility, allowing patients to stay on treatment, while returning to a more ‘normal’ life.18 In our experience, patients tend to present at the clinic regularly for the first 3–6 months of treatment with depot buprenorphine, but then space the injections out, possibly with a view to stopping MATOD. We intend to follow up the 8% of patients who had planned discontinuation from treatment in a subsequent study.
Buprenorphine is a partial opioid agonist. One of the risks with commencement of buprenorphine is precipitated withdrawal. This is characterised by rapid onset of withdrawal symptoms and is a recognised side effect of buprenorphine use.1 It occurs when buprenorphine rapidly displaces full opioid agonists, particularly long-acting opioids, such as methadone and oxycodone MR, already present on the mu opioid receptor. Patients already on buprenorphine in this clinic were asked to take their last sublingual dose 10–15 minutes before the first injection of depot to avoid precipitation, should they recently have missed any doses of sublingual buprenorphine and recently used any full agonist opioids.
During early experience with depot buprenorphine at this clinic, many patients experienced some pain or discomfort during the subcutaneous injection of the 300 mg depot, to the extent that they were reluctant to continue treatment. Therefore, to help prevent patients from dropping out of treatment because of painful injections, the clinic adopted a standard practice of administering local anaesthetic to the injection site first. No negative consequences or adverse effects were observed using this approach, and it could help retain patients in treatment.
This is a small observational retrospective study in a single general practice in a metropolitan-suburban setting with a group of experienced MATOD prescribers. Most of the patients were stable in treatment and had a history of heroin use. This could limit generalisability to patients new to treatment, not injecting drugs or with prescription opioid use disorder. However, the study population was largely consistent with the national profile of patients being treated for opioid dependency,11 although a higher proportion were dependent on heroin. As the aim of the study was to record retention in treatment, not abstinence rates, urine drug screens were not conducted; therefore, it is not possible to comment on patients’ abstinence during treatment or rates of relapse after cessation of treatment. Findings might have been confounded by the COVID-19 pandemic restrictions and changes to prescribing of buprenorphine and methadone during this time. Broader cross-sectional research will be needed to determine retention in a range of settings and patients, including patients dependent on prescription opioids. The Community Long-Acting Buprenorphine study (no.: NCT03809143) is investigating the real-world implementation of monthly buprenorphine in general practice and specialist drug treatment services in Australia, and includes qualitative research and costing, as well as clinical outcomes.19 It is important to identify likely reasons for discontinuation of monthly buprenorphine among patients in the general practice setting, and how this might be avoided. While retention in treatment is important, this needs to be considered alongside additional clinical benefits, particularly abstinence and inhibition of cravings, and patients’ personal treatment goals.
Conclusion
This real-world study in a metropolitan-suburban general practice setting demonstrates that monthly buprenorphine depot, with some flexibility in dosage interval, retained more than half of patients on treatment for approximately six months.