The cornea is the transparent anterior covering of the eye and comprises the epithelium, Bowman’s layer, stroma, Descemet’s membrane and endothelium (Figure 1). The epithelium serves as the principal barrier to infection.1 A corneal abrasion is a defect in the surface epithelium. Small epithelial abrasions generally heal rapidly and without sequelae and will not be further discussed in this article. In contrast, a corneal ulcer is a defect in the surface epithelium and underlying stroma (Figure 1). Significant morbidity can follow corneal ulceration, with complications including corneal scarring and/or perforation, glaucoma, cataract and loss of vision. In this article, the authors present a case and management guide for general practitioners (GPs) with patients who present with reduced vision due to corneal ulceration.
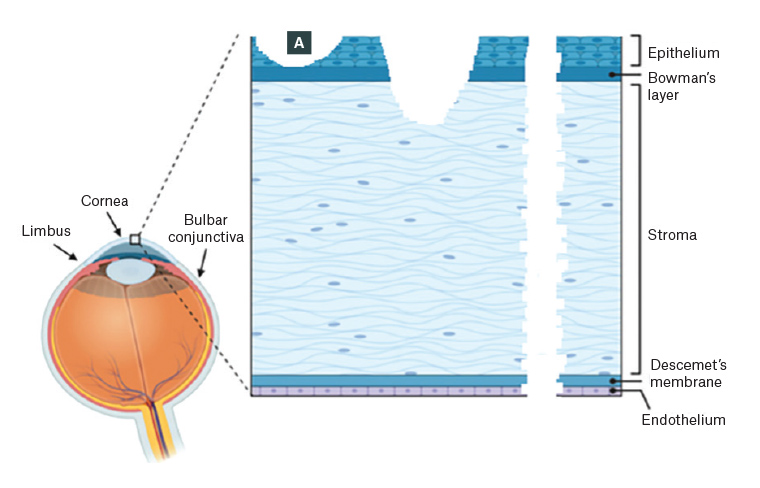
Figure 1. Schematic showing corneal layers and injuries
A. Corneal abrasion involving epithelium only; B. Corneal ulceration involving epithelium, Bowman’s layer and underlying stroma; C. Corneal full-thickness perforation, which can occur following fulminant infectious ulceration or in trauma, where a foreign body may be present in the cornea or anterior chamber
Reproduced from Zhang X, Mélik-Parsadaniantz S, Baudouin C, Réaux-Le Goazigo A, Moreau N. Shhedding new light on the role of hedgehog signaling in corneal wound healing, Int J Mol Sci;23:3630. doi: 10.3390/ijms23073630. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0).
Case
A man aged 19 years presented to a tertiary ophthalmic centre with a sore, painful left eye. Three days earlier he had swum in a freshwater lake, while wearing his contact lenses, to retrieve his dropped mobile phone. He presented with deteriorating vision and increasing pain. He regularly showered and slept with contact lenses in situ.
On examination, best visual acuities were right 6/18 pinhole, left perception of light. The left cornea had a stromal infiltrate with overlying epithelial defect (corneal ulcer) with a 0.5 mm hypopyon. The conjunctiva was oedematous and injected (Figure 2). The remaining examination was normal.
He had a corneal scrape and was treated with topical ofloxacin 0.3% once per hour day and night. The scrape grew Pseudomonas aeruginosa, which was sensitive to ofloxacin, tazobactram and tobramycin but resistant to chloramphenicol. Treatment was tapered over six weeks. Final best corrected visual acuity was 6/48 because of corneal scarring. A corneal graft was to be considered after a minimum of 12 months.
P. aeruginosa is a ubiquitous organism, often found in natural waters such as rivers. Contact lens use is a major risk factor for Pseudomonas spp. keratitis as the organisms can reside on the posterior surface of the lens, protected from host defence mechanisms. Red flags in this case were swimming while wearing contact lenses, the continued use of lenses while the eyes were sore and reduced vision pointing to the need for a corneal scrape prior to institution of treatment for infectious keratitis.
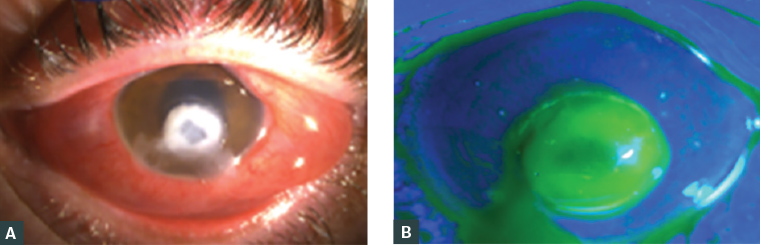
Figure 2. The left eye of a man aged 19 years with Pseudomonas spp. keratitis and visual acuity perception of light
A. Anterior segment photograph showing a dense infiltrate and conjunctival oedema and injection; B. Blue light imaging after application of fluorescein demonstrating overlying epithelial defect extending beyond the region of infiltrate. Hypopyon (pus in the anterior chamber) was present but not well demonstrated in the images.
Case data supplied by Dr Colby T Hart.
Aetiology of corneal ulceration
The most vision-threatening causes of corneal ulceration are trauma and infection (infectious or microbial keratitis). Neurotrophia, exposure, inflammation (marginal keratitis) and rarely autoimmune conditions are further causes, although these are usually less acutely vision threatening.
Trauma
Trauma usually presents in an acute setting, particularly after hammering and grinding, which can result in high-energy foreign bodies. Pouring chemicals can lead to splash injuries to the face and eyes; acids and alkali can penetrate deeper into the cornea and produce an ulcer. Direct trauma with sharp objects, assault and accidents may cause ulceration, and in some cases cause corneal perforation (Figure 1). GP management of trauma was recently comprehensively reviewed.2
Infectious keratitis
An infection to the cornea may be due to bacteria, viruses or fungi and can be sight threatening.3 Common risk factors for all organisms include contact lens wear, particularly with poor hygiene; previous trauma or ocular pathology disrupting the corneal epithelial barrier; and topical corticosteroid use. GP management of infectious keratitis was recently reviewed.4
Bacterial keratitis is the most common cause of microbial keratitis and an ophthalmic emergency that requires immediate treatment to prevent visual loss. The most common causative bacteria in Australia are gram-positive coagulase-negative Staphylococci and Staphylococcus aureus.5,6 Gram-negative P. aeruginosa is associated with contact lens wear. It can penetrate intact corneal epithelium, leading to bilateral keratitis with endophthalmitis, and can result in poor clinical outcomes with long and costly treatments.7,8
Herpetic eye disease may have a recurrent pattern and be associated with other ocular findings in any part of the eye, including lid vesicles and conjunctiva anteriorly to posterior segment changes. Herpes simplex virus (HSV) is the most common cause of herpetic keratitis and the most common cause of corneal blindness in the developed world.9 Varicella zoster virus (VZV) and cytomegalovirus (CMV) are less common causes.
Fungal keratitis is more common in hot and dry climates, agricultural communities and after trauma with vegetative matter. In Australia, Candida, Aspergillus and Fusarium spp. commonly cause fungal keratitis.10,11
Acanthamoeba spp. are ubiquitous organisms found in the environment in fresh water, soil and air. Keratitis due to Acanthamoeba spp. is commonly associated with contact lens wear, particularly with poor hygiene practices.12 It is typically painful and requires prolonged treatment if not diagnosed early.13
Less acutely vision-threatening causes
Neurotrophia
Neurotrophic corneal ulcers are due to reduced corneal sensation secondary to damage to the ophthalmic branch of the trigeminal nerve. The most common cause is HSV/VZV infections. Less commonly neurotrophia is due to corneal surgery, fifth nerve compressive lesions and diabetes mellitus. People with neurotropic ulcers typically present with a prolonged history. Loss of corneal sensation will be found on examination.
Corneal exposure
In severe cases, exposure keratopathy can lead to corneal ulceration and can occur with lagophthalmos, typically due to facial nerve palsy or lid abnormalities such as ectropion.
Marginal keratitis
Marginal keratitis is a common inflammatory condition of the peripheral cornea that begins with an infiltrate that then ulcerates the overlying epithelium. It is usually associated with blepharokeratoconjunctivitis and is thought to be an inflammatory response to S. aureus antigens on the lid margins.
Autoimmune disease
Peripheral ulcerative keratitis (‘corneal melt’) is a rare condition that may be associated with rheumatoid arthritis, collagen vascular disease, systemic lupus erythematosus and granulomatosis with polyangiitis (Wegener’s), among other conditions. The course may be indolent, with only mild pain and ocular inflammation.14,15
History and clinical examination
Most GPs have an ‘eye kit’: Snellen chart, pinhole occluder, topical anaesthetic drops (eg oxybuprocaine hydrochloride 0.4%), direct ophthalmoscope or pen torch (with a blue light source), and fluorescein solution or strips. Slit lamps are useful, if available.4
History should focus on determining if vision is reduced and the time course of any reduction in vision, extent of pain and overall duration of symptoms, as well as elucidating risk factors. Reduced visual acuity is an indicator of disease severity.16 Trauma and infections tend to present acutely, but a prolonged course increases the likelihood of corneal ulceration as opposed to abrasion. Risk factors for ulceration – such as contact lens use (particularly while swimming or showering), recent trauma, hammering or grinding, chemical injury (elucidate the acid or alkali nature of the agent if possible), exposure to vegetable matter (eg potting mix) and history of herpes (labial or ocular) – should be sought. Significant systemic conditions include immunosuppression, diabetes mellitus and autoimmune conditions.
Examination begins with visual acuity. The face and eyelids are evaluated for blepharitis (lid margin erythema, meibomian gland plugging and/or lash debris), herpetic vesicles, facial nerve palsy, eyelid abnormalities and/or trauma. All patients are evaluated for foreign bodies, including subtarsal eversion, and corneal perforation is considered. Conjunctival oedema and erythema suggest an acute condition. Corneal sensation should be checked using the tip of a tissue prior to application of local anaesthetic. Confrontation visual field may be assessed if clinically relevant (eg suspected neurological condition with neurotrophic cornea).
The cornea is inspected for epithelial defects and then evaluated with fluorescein dye. Corneal epithelial defects appear green using the cobalt blue light on the ophthalmoscope or slit lamp. The shape and location of the epithelial defect can give clues to the diagnosis. Traumatic defects may have ragged edges initially, which then heal from the margins, leading to a stellate pattern of healing (Figure 3A). Bacterial keratitis typically has an epithelial defect larger than the stromal infiltrate (Figure 2B). HSV keratitis typically has a dendritic (branching) staining pattern (Figure 3B).17 Neurotrophic ulcers are typically an oval-shaped defect in the inferior or central cornea with rolled edges (Figure 3C). Marginal keratitis lesions typically have a small epithelial defect overlying a larger stromal opacity and are located close to, but separate from, the limbus, often at 1, 5, 7 and 11 o’clock, where the eyelid contacts the corneal limbus (Figure 3D). Autoimmune ulceration typically is larger than marginal keratitis, crescentic in shape, close to but distinct from the limbus, and with associated local conjunctival injection, which aids in differentiating it from arcus senilis (Figure 3E). Slit lamp examination of autoimmune ulceration reveals an undermining edge. Acanthamoeba keratitis typically has a ring-shaped infiltrate (Figure 3F).13 Corneal vascularisation (Figure 3G) suggests an acute on chronic condition, such as recurrent HSV.
By definition, corneal ulcers involve the corneal stroma, which should be inspected with a direct ophthalmoscope or pen torch for oedema (diffuse haze, which can obscure anterior chamber details [Figure 3G]) or focal infiltrate (dense white opacity [Figures 2A, 3G]). Infiltrates with fluffy borders and satellite lesions are suggestive of fungal infection.
Finally, the anterior chamber is inspected. A hypopyon suggests infectious keratitis with anterior chamber involvement (Figure 3F); hyphaema suggests trauma. In chemical injuries, the ability to identify intraocular structures such as the iris can help to indicate severity – the hazier the view, the worse the injury (Figure 3H). The depth and nature of stromal and anterior chamber involvement is best appreciated with slit lamp examination, if available. If corneal ulceration is suspected and no slit lamp is available to confirm the diagnosis, referral may be required to ensure adequate examination and management.
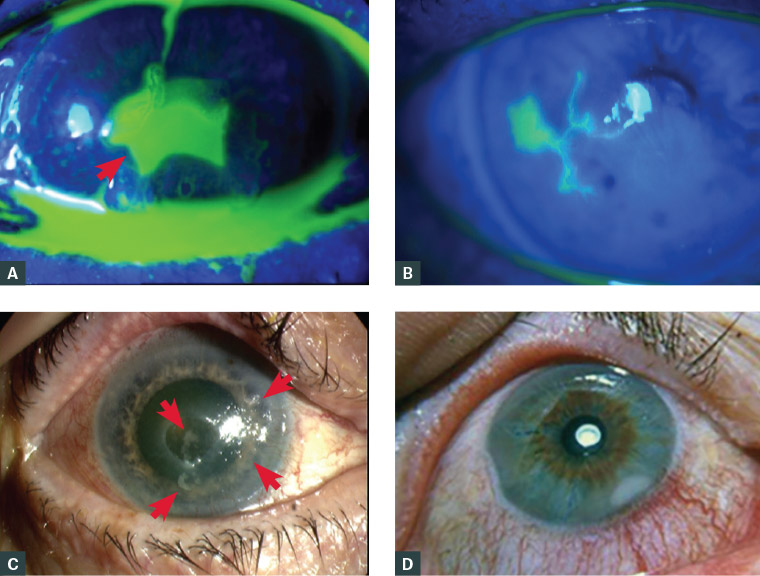
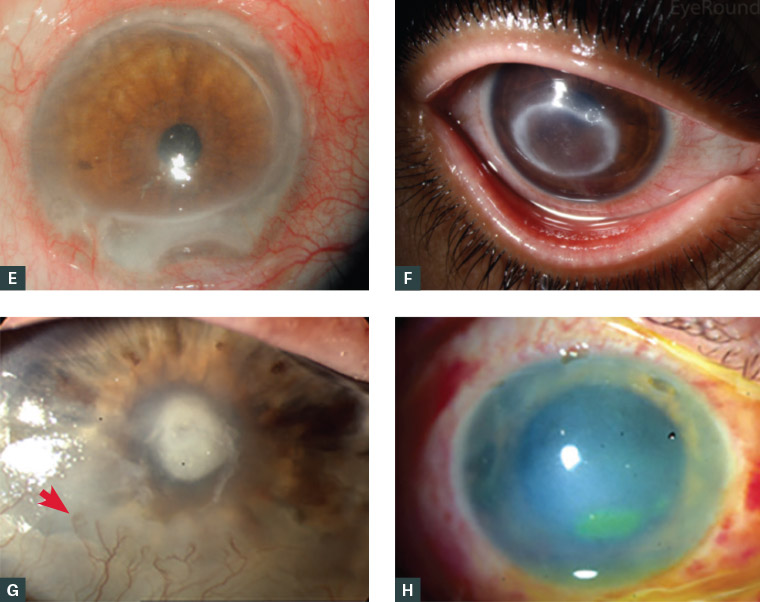
Figure 3. Anterior segment photographs
A. Healing corneal abrasion showing stellate pattern of fluorescein staining due to epithelial ingrowth from the margin (arrow); B. Fluorescein staining of dendritic ulcer in herpes simplex keratitis; C. Neurotrophic corneal ulcer with smooth, rolled edges (arrows delineate the ulcer); D. Marginal keratitis in the corneal periphery, separated from the limbus, with local conjunctival injection; E. Peripheral ulcerative keratitis with crescentic destruction of juxtalimbal corneal stroma; F. Ring-shaped stromal opacity in Acanthamoeba keratitis; G. Bacterial keratitis with corneal ulcer, with the opacity (infiltrate) in the base of the corneal ulcer and corneal neovascularisation (arrow) suggesting acute-on-chronic pathology; H. Corneal ulcer stained with fluorescein in a cloudy cornea (corneal haze) following an alkali injury
Figure 3D has been reproduced from Stiff AH, Ricca AM, Goins KM, Corneal marginal ulcer: Marginal keratitis with ulceration in a 45 year-old male, Iowa City, IA: The University of Iowa, 2017. Available at www.EyeRounds.org/cases/249-corneal-marginal-ulcer.htm (licensed under CC BY-NC-ND 3.0 [https://creativecommons.org/licenses/by-nc-nd/3.0/deed.en_US]).
Figure 3F has been reproduced from EyeRounds.org, Acanthamoeba keratitis, Iowa City, IA: The University of Iowa, [date unknown]. Available at https://webeye.ophth.uiowa.edu/eyeforum/atlas/pages/acanthamoeba/index.htm (licensed under CC BY-NC-ND 3.0 [https://creativecommons.org/licenses/by-nc-nd/3.0/deed.en_US]).
Differential diagnoses
The differential diagnosis of corneal ulceration is corneal abrasion involving the corneal epithelium only. Differential diagnoses of a red, painful eye with reduced vision (but without corneal ulceration) include acute angle closure glaucoma, anterior uveitis, episcleritis and scleritis.
Management by general practitioners
Corneal ulceration due to trauma (particularly where corneal perforation [with or without foreign body] cannot be excluded), alkali or acid injuries and all cases of infectious keratitis require urgent ophthalmology referral. Chemical injuries require copious irrigation with normal saline, and suspected infectious keratitis requires removal of contact lenses by the patient or GP prior to referral. It is important to not treat infectious keratitis empirically prior to referral, as this confounds the microbiological analysis that is required to direct treatment. Urgent ophthalmology review is also required for abrasions that do not heal within a couple of days. These conditions are time critical, and direct personal liaison with the ophthalmologist and/or tertiary ophthalmic centre is required.
GP management of other causes of corneal ulceration is summarised in Table 1. Herpes simplex involving epithelium only is treated with topical antivirals, but ophthalmic referral for the first episode can be considered to allow corneal sampling for polymerase chain reaction (PCR) confirmation of diagnosis and to direct future treatment. GPs treating herpes zoster ophthalmicus play a part in sending skin vesicle fluid for PCR confirmation (in cases of diagnostic uncertainty) and starting oral antivirals with 72 hours of skin vesicle onset to reduce post-herpetic neuralgia. If corneal ulceration develops, ophthalmology referral is required for definitive treatment. In indolent cases of corneal ulceration, it is important that GPs have a high index of suspicion of fungal keratitis (in people with traumatic vegetation exposure to the cornea) and Acanthamoeba keratitis (where the eye is painful out of proportion to the signs); suspicion of these diagnoses should prompt urgent referral.
| Table 1. Treatment prescribed by general practitioners for corneal ulcers |
| Diagnosis |
General practitioner management |
Ophthalmologist management |
| Herpes simplex keratitis19,20 |
Ophthalmic acyclovir 3% ointment five times per day for 14 days for epithelial keratitis without stromal ulceration.
|
Oral valaciclovir* 1 g TDS for 7–10 days, or topical acyclovir and steroids for corneal ulceration. Further treatment depends on specific intraocular involvement and degree of immunosuppression. |
| Herpes zoster keratitis (herpes zoster ophthalmicus) |
Oral valaciclovir* 1 g TDS if within 72 hours of rash onset.
Ophthalmic acyclovir 3% ointment five times per day for 10 days for epithelial keratitis. No role in prophylaxis at presentation with herpes zoster ophthalmicus. |
Further treatment depends on specific intraocular involvement and degree of immunosuppression. |
| Neurotrophia |
Ointment lubricant, such as hypromellose 3 mg/g, carbomer 980 2.2 mg/g QID. |
May require tarsorrhaphy, autologous serum drops
and/or amniotic membrane transplantation. |
| Marginal keratitis |
Chloramphenicol eyedrops 0.5% QID for five days.
Treatment of underlying blepharitis. |
May require topical steroids. |
| Autoimmune keratitis |
Consider rheumatology referral for autoimmune screen and diagnosis. |
May require topical steroids, systemic immunosuppression and corneal grafting. |
*Doses of oral valaciclovir are reduced in people with renal disease and paediatric patients. Use of topical acyclovir is considered safe during pregnancy.21 Use of oral antivirals in the first trimester of pregnancy is not associated with birth defects. Acyclovir is preferred because of more clinical experience (Category B3). Valaciclovir is considered second line and may be used from 36 weeks’ pregnancy (Category B3).22
TDS, three times per day; QID, four times per day |
Investigation by ophthalmologists
Imaging may be required in trauma to rule out foreign bodies. In infectious keratitis, the clinical appearance can be similar for different causal organisms, and sampling of the cornea by ophthalmologists for microscopy and culture and/or PCR detection is essential prior to treatment initiation.18
Prognosis
Prognosis is dependent on the etiology, severity and location of the corneal ulcer (central vs peripheral) and prompt and appropriate treatment. In cases with healthy limbal stem cells, normal corneal sensation and minimal damage to the corneal stroma, corneal ulcers generally heal with appropriate therapy, though vision may still be affected.
Patient education
The importance of safety eyewear should be emphasised to patients at risk of corneal foreign bodies. People who wear contact lenses should be educated on their proper use and handling, avoid swimming and showering in contact lenses and avoid buying lenses from nonmedical sources. People being treated for ocular conditions, such as corneal abrasions, need to understand the importance of reduction of vision and/or increasing pain in prompting urgent review.
Conclusion
Red flags for corneal ulceration include severe loss of vision; trauma, hammering or grinding; chemical splashes; and contact lens use, particularly with poor hygiene practices. Urgent referral to ophthalmologists is needed for corneal ulceration from trauma, including chemical injuries, and suspected corneal infections. Direct liaison with the ophthalmologist is required to ensure appropriate clinical transfer of care. GPs play a part in managing other causes of corneal ulceration, with referral to ophthalmologists within 2–3 days if required, and rheumatologists if systemic autoimmune disease is suspected.
Key points
- Corneal ulcers are deeper than abrasions and involve the corneal stroma.
- Corneal ulcers have multiple aetiologies but typically cause reduced vision, pain and a red eye.
- Trauma and suspected microbial keratitis require urgent ophthalmology referral.