This article is the seventh in a series of articles on important topics in neurology.
Parkinson’s disease is a neurodegenerative disorder characterised by slow progression over years. Symptom profile and treatment response guide classification as early, mid and late stage (Figure 1). In early disease, although non-motor symptoms may be present and often precede diagnosis, levodopa-responsive motor symptoms predominate, and most patients are effectively managed with oral medications.1 Motor fluctuations become prominent in mid-stage Parkinson’s disease; the therapeutic window narrows, and patients may be considered candidates for device-assisted therapies (Figure 2; Table 1). Surviving patients enter late-stage Parkinson’s disease (LSPD), where there is a shift to increasing disability from non-motor symptoms unresponsive to levodopa (Figure 1).2 In addition to the LSPD milestones of frequent falls (Table 2), cognitive impairment, visual hallucinations and need for residential care,3,4 patients encounter dysarthria, hypophonia, dysphagia, constipation, bladder dysfunction, pain and neuropsychiatric disturbances.5 These may be more disabling than motor symptoms. Treatment goals must therefore be individualised to ensure patient comfort and quality of life while minimising caregiver burden. The onset of LSPD milestones is a harbringer of faster disease progression and predicts death, but variability in disease course is well recognised.
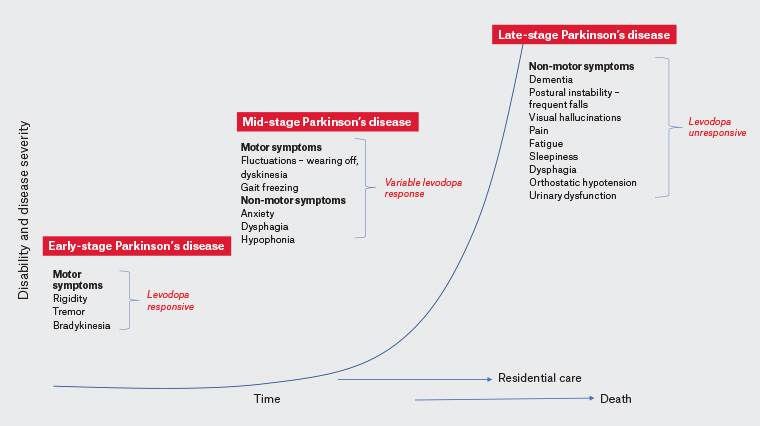
Figure 1. Exponential increase in disease severity and disability is seen in late-stage Parkinson’s disease with disability milestones of dementia, regular falls, the need for residential care and visual hallucinations predictive of a poor prognosis. A selection of pertinent clinical features by stage are listed. Click here to enlarge.
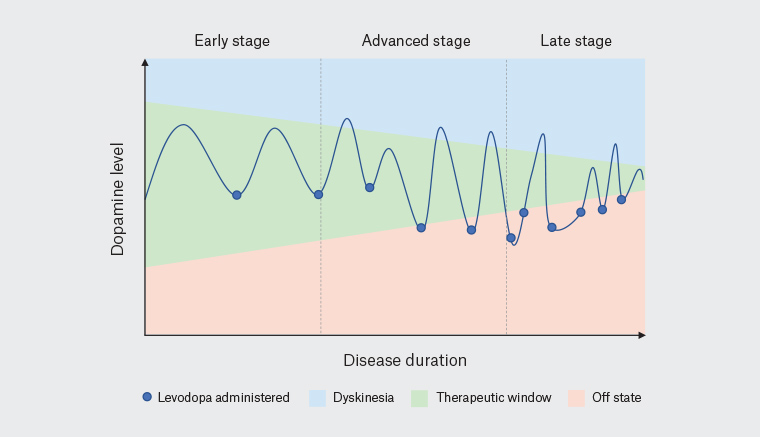
Figure 2. The therapeutic window in Parkinson’s disease narrows with progression through disease stage.
| Table 1. Advanced therapies for mid-stage Parkinson’s disease |
| Advanced therapy |
Utility |
Advantages |
Potential complications |
Other considerations |
| Apomorphine |
Intermittent subcutaneous rescue therapy for ‘off’ periods OR
Continuous infusion for reducing dyskinesia, motor fluctuations and oral levodopa |
Rapid onset
Procedure free
Easy to discontinue
Initiated in outpatient setting |
Injection site skin nodules
Nausea
Orthostatic hypotension
Neuropsychiatric issues |
Medication-induced haemolytic anaemia |
| Levodopa continuous intestinal gel |
Continuous infusion to jejunum (bypasses delayed gastric emptying) via PEG-J mimicking physiological dopaminergic levels |
Near-complete replacement of oral dopaminergic therapy
|
Device maintenance and tube issues (dislodgement, kinking, infection, bezoar) |
Carer training and assistance
Peripheral neuropathy (biochemical and neurophysiological monitoring)
Cosmesis |
| Deep brain stimulation |
Stereotactic insertion of electrodes to deliver electrical stimulation to basal ganglia |
Non-destructive and facilitates bilateral treatment when compared with irreversible lesioning techniques (eg magnetic resonance–guided focused ultrasonography)
Superior for medication refractory tremor |
Perioperative complications
Worsening dysarthria and apathy
Device-related infections |
Worse outcomes with older age (>75 years), cognitive impairment, levodopa unresponsive symptoms (postural instability)
Ongoing programming to optimise response required
|
| PEG-J, percutaneous endoscopic transgastric jejunostomy |
| Table 2. Factors contributing to falls in late-stage Parkinson’s disease |
| Factor |
Mechanism |
Possible actions |
| Orthostatic hypotension |
Lightheadedness, weakness, fatigue, syncope |
|
| Postural instability |
Loss of postural reflexes |
- Physiotherapy
- Walking aids
- Carer supervision
|
| Freezing of gait |
Multifactorial – including variable medication effect |
- Optimise antiparkinsonian medications
- Consideration of continuous therapies (device assisted) with specialist neurology services
|
| Cognitive decline |
Attention deficits, reduced safety awareness, impulsivity |
- Carer supervision and redirection
- Rationalise medications, including dopamine agonists, contributing to impulsivity
|
| Delirium |
Multifactorial |
- Investigate and treat underlying cause
|
| Somnolence |
Sedation, fatigue,
sleep attacks |
- Reduce sedating medications
- Enquire regarding sleep attacks
- Consider referral for sleep studies – query central or obstructive sleep apnoea
- Consider modafinil
- Driving safety screen
|
| Table 3. Common chronic symptoms in late-stage Parkinson’s disease and suggested management |
| Symptom |
Approach and treatment options |
Constipation
|
- Dietary fibre and hydration
- Aperients
- Bulk forming (regular dietary fibre supplements)
- Osmotic (regular macrogel, 1–2 sachets twice daily)
- Stimulant (short-term use only of senna, bisacodyl)
- Softeners (regular docusate sodium)
|
| Nausea, satiety |
- Short courses of domperidone (consider risk of QT prolongation)
- Avoid dopamine blockade: metoclopramide, prochlorperazine
|
Sialorrhoea
|
- Sublingual atropine eye drops 1% (1–2 drops QID)
- Sublingual ipratropium inhaler (two puffs QID)
- Specialist neurology referral for botulinum toxin
|
| Urinary frequency, nocturia and incontinence |
- Amitriptyline 12.5–50 mg at night
- Solifenacin controlled release 5–10 mg/day
|
| Orthostatic hypotension |
- Non-pharmacological
- Hydration – at least 1.5 L/day
- Increase salt intake
- Compression stockings
- Avoid large meals, warm environment
- Slow change in posture; head up tilt of bed at night 30–40°
- Pharmacological
- Short-acting pressors; fludrocortisone 100–300 µg in divided doses morning and midday, midodrine 2.5–10 mg; avoid evening administration because of risk of supine hypertension
- Pyridostigmine 30–60 mg TID
- Avoid exacerbating medications: diuretics, nitrates, antihypertensives
- Consider 24-hour blood pressure monitor – may identify nocturnal hypertension
- Pressor prior to levodopa to mitigate hypotensive effect
|
| Depression |
- Psychosocial support, social engagement
- Pharmacological
- Venlafaxine SR, paroxetine and sertraline
- Mirtazapine 15 mg at night may aid insomnia; higher doses lose sedative effect but treat depression
|
| Apathy |
- Rotigotine patch (commence 4 mg/24 hrs)
- Rivastigmine patch (commence 4.6 mg/24 hrs)
|
| Insomnia |
- Nocturnal akinesia – optimise nocturnal dopaminergic medications
- RLS – daytime exercise earlier in the day, supplement iron deficiency, evening dopamine agonist, pregabalin
- RBD – clonazepam (starting dose 0.25–0.5 mg at night)
- Above excluded – mirtazapine 15 mg, amtiriptyline 12.5–25 mg
|
| Daytime somnolence |
- Screen for obstructive/central sleep apnoea and insomnia
- Cease daytime sedatives
- Consider sleep attacks from dopamine agonists
- Treat hypotension (refer to above)
- Regular medication timings – avoid reduced attention and alertness seen in ‘off’ state or ‘dyscognitive off’
- Above excluded – trial modafinil
|
| QID, four times per day; RBD, rapid eye movement sleep behaviour disorder; RLS, restless legs syndrome; SR, slow release; TID, three times per day |
The aim of this article is to outline these challenges and arm the general practitioner (GP) with practical tools for the daily management of patients with LSPD.
Motor aspects of late-stage Parkinson’s disease
In LSPD, the majority of motor symptoms remain responsive to medication, but the peak benefit may decline.3 Escalating doses of dopaminergic medications may not achieve optimal improvement and may induce adverse effects. To achieve the goals of maintaining comfort and reasonable function without inducing unwanted effects, some form of dopaminergic therapy should be continued as dysphagia, bradykinesia and rigidity remain treatment responsive. Device-assisted therapy (Table 1) should also generally be continued in LSPD. Patients with disability continue to benefit from allied health input to maintain function and pressure area integrity and avoid contractures and injuries. Commonly used strategies for motor fluctuations are discussed elsewhere;6 however, in LSPD, troublesome dyskinesia is uncommon.3
Non-motor aspects of late-stage Parkinson’s disease
Non-motor symptoms predominate in patients with LSPD, and management shifts to addressing cognitive decline and neuropsychiatric complications, somnolence, postural hypotension, gastrointestinal dysmotility, dysphagia, nutritional challenges and pain (Table 3).3 As more patients reside in residential care facilities, they increasingly depend on their GPs for medical care (Table 4).
| Table 4. Care of patients with late-stage Parkinson’s disease in residential care |
| General practitioner interventions |
Nursing interventions |
Allied health |
- Regular review is crucial
- Continue regular neurology specialty appointments – in‑person/telehealth/outreach
- Continue device-assisted therapies – specialist services on hand for advice
- Prompt treatment of systemic illness
- Regular review of mood – watch for signs as patients may not report depression
- Consider community palliative team involvement
|
- Consistent timings of Parkinson’s disease medications
- Optimise feeding and mobilisation schedule for ‘on’ effect of medications
- Bowel maintenance; 1–2 soft motions per day
- Monitor pressure areas
- Toileting regularly (every 2–3 hours) may help prevent incontinence and urinary tract infections
- Regular contact with specialist neurology nursing
- reaffirm patient-specific priorities
- update staff education – note patients receiving device-assisted therapies
- Monitor nutritional status
- Ensure adequate supply of specialist Parkinson’s disease medications on site – especially important during public holidays
- Ensure Webster pack is up to date
- Awareness of contraindicated medications
|
- Physiotherapy – even in patients who are severely disabled to maintain ability to transfer or mobilise short distances, prevent complications of immobility
- Occupational therapy – positioning and splinting to prevent painful contractures and pressure ulcers
- Regular dietetics review
|
Cognitive decline in Parkinson’s disease
The occurrence of dementia associated with Parkinson’s disease (PDD) correlates strongly with disease duration. Eighty-three per cent of those who survive 20 years following diagnosis will experience PDD.7 Recognition and timely management are paramount to maintaining patient comfort and minimising caregiver stress.
Recognising cognitive decline in Parkinson’s disease
PDD and dementia with Lewy bodies (DLB) are characterised by day-to-day, or even hour-to-hour, fluctuations in cognition, attention and alertness. Periods of lucidity alternate with confusion, which contrasts with the omnipresent progressive amnesia of Alzheimer’s disease. Visual hallucinations are prominent, occurring in up to 90% of patients with Parkinson’s disease,8 and are a risk factor for progression to PDD but may occur in its absence. Early warning signs include impaired light/shade discrimination, difficulty judging distance, extracampine hallucinations (including a presence in the peripheral visual field) and visual illusions. Lilliputian hallucinations are frequently described, consisting of small animals or people. Hallucinations are more prominent at night or on wakening. It is recommended to screen for disorders of visual impairment that may contribute to hallucinations (eg diabetic retinopathy, macular degeneration or cataracts). Relatively slow progression and the sequence of symptom onset are of diagnostic importance in order to distinguish PDD from DLB, where a dementing illness occurs prior to, or concurrently with, the development of motor symptoms of Parkinsonism.9 Asking patients about cognitive concerns, collateral history and formal assessment are important steps at all disease stages. The Montreal Cognitive Assessment is a more sensitive screening tool than the Mini-Mental State Examination for Parkinson’s disease.
Treatment approach to neuropsychiatric manifestations of late-stage Parkinson’s disease: Prevention
Active measures to avoid known reversible causes of delirium is of paramount importance in LSPD. These authors advise regularly using an aperient to prevent constipation (Table 3), promptly treating infections, maintaining a consistent medication schedule and avoiding anticholinergics, sedatives and opiates. While seemingly obvious, these four factors, either combined or in isolation, are among the most common reasons for preventable deteriorations and lengthy hospital admissions. Parkinson’s medications can exacerbate cognitive symptoms and can also precipitate hallucinations and delusions. Levodopa alone is far less likely to result in such complications. An approach to medication rationalisation is discussed elsewhere.6
Psychosis and hallucinations in Parkinson’s disease
In addition to visual hallucinations and illusions, PDD/DLB may be associated with psychotic symptoms such as complex delusions, paranoia and agitation.10 Antipsychotics with greater dopamine antagonism (haloperidol, olanzapine, risperidone) should be avoided.10,11 Clozapine is the most effective medication for PD psychosis (PDPsy), and low doses (up to 50 mg daily) are effective.11 Its routine use is limited by the rare but serious non–dose related complication of agranulocytosis, necessitating regular laboratory monitoring. In Australia, a registered prescriber and psychogeriatrician referral is typically required. Evidence for efficacy of quetiapine is less definitive, but its favourable side effect profile lends itself to frequent use.11 These authors trial quetiapine first, commencing at low doses (eg 12.5 mg or 25 mg at night) and uptitrating in similar increments according to response. Potential side effects include sedation, falls, orthostatic hypotension and QT prolongation. Atypical antipsychotics should be used with caution because of the risk of falls, cardiovascular effects, stroke and death.12 Pimavanserin (a 5HT-2A receptor inverse agonist) has proven efficacy in PDPsy but is not yet routinely available in Australia.
Cholinesterase inhibitors (specifically rivastigmine and donepezil) may be useful for both psychotic and cognitive complications. Although only licensed for Alzheimer’s disease in Australia, many patients with PDD/DLB have Alzheimer’s pathology in addition to Lewy bodies, and clinical differentiation between the two is not always reliable. Clinical evidence supports the use of cholinesterase inhibitors in Parkinson’s disease–related cognitive impairment.13 Anecdotally, it is most effective for reducing attentional fluctuations, as cholinergic denervation in Parkinson’s disease has been associated with memory, attention and executive dysfunction. Rivastigmine has the added benefit of transdermal delivery.
LSPD is also associated with broader neuropsychiatric disturbances including depression, anxiety, apathy, impulsive and compulsive behaviours. Depression may be present in up to 35% of patients with Parkinson’s disease in residential care and profoundly affects quality of life.14 Regular screening is appropriate, and remaining alert to evolving anorexia, weight loss, fatigue, altered sleep, unexplained motor or cognitive deterioration, irritability, feelings of guilt and inadequacy and pessimism about the future may facilitate diagnosis. Depression and anxiety may fluctuate and occur during ‘off’ periods. Although no clinical trials have been performed in LSPD, these authors have a low threshold for commencing selective serotonin reuptake inhibitors, with the best evidence in favour of venlafaxine and paroxetine,12 although in these authors’ experience sertraline can also be very effective, especially when there is a strong component of anxiety. Involvement of family members,15 social engagement and sleep hygiene should be encouraged.
Apathy is prevalent in LSPD, correlating with executive dysfunction and heralding dementia.16 Differentiating depression, apathy, demoralisation and cognitive deficits is challenging but can help tailor treatment. Apathy is characterised by a blunted emotional response with a reduction in enthusiasm and goal-directed behaviours. These authors have found cautious use of dopamine agonists beneficial (eg rotigotine patch 4 mg/24 hr). Rivastigmine has some proven efficacy.17 Antidepressants and stimulants, such as methylphenidate and the norepinephrine–dopamine reuptake inhibitor bupropion, may be trialled under specialist psychiatric supervision. In contrast, low motivation associated with depression or dementia stem from emotional distress or cognitive impairment, respectively. Depression can be associated with anxiety, agitation and irritability; features typically lacking in apathy. Demoralisation refers to feelings of helplessness, hopelessness and a sense of failure and has been linked to decreased functionality and quality of life in Parkinson’s disease.18 Treatment of demoralisation centres on psychotherapeutic and behavioural therapies.
Late-stage Parkinson’s disease and the gastrointestinal tract
Constipation is underrecognised by patients and is worth devoting time to during consultations. Constipation often worsens in LSPD because of autonomic denervation and declining mobility, precipitating acute motor and cognitive deterioration, nausea and abdominal discomfort. Proactive management (Table 3), including enemas if necessary, aims to maintain 1–2 soft bowel motions per day.1 In those receiving levodopa carbidopa intestinal gel or enteral feeding, macrogol may be administered through the gastric port following training with a specialist neurology nurse or dietitian.
Nutrition and swallowing
Progressive dysphagia affects nutritional status and can cause aspiration pneumonia and distress. Frequent screening for weight loss, choking episodes and oral intake is recommended. Dysphagia may be partially levodopa responsive. Adapting medication schedules so that patients are ‘on’ at mealtimes can help reduce aspiration risk (consider oral levodopa dose 30 mins pre-meals). Speech pathology assessment can facilitate early detection and rehabilitation. In those failing to meet their nutritional requirements orally, expert consensus recommends consideration of enteral feeding with a case-by-case, individual risk–benefit evaluation.19 Consequences of poor nutrition include fatigue, infection, pressure ulcers and reduced survival. Eating and drinking remain integral to quality of life and comfort, and patients may decide to acknowledge the risk of aspiration and maintain some oral intake. This should be respected if the risks are clearly understood, and treating neurologists are on hand to provide documentation to this effect if required. Tips to aid medication administration include using a pill crusher and mixing with food, dissolving medications (refer to manufacturer guidelines) or converting to a transdermal dopamine agonist preparation at the end-of-life stage. Apomorphine intermittent injections may also be appropriate with specialist neurology input.
Orthostatic hypotension
Orthostatic hypotension is almost universally encountered in LSPD, manifesting as lightheadedness, weakness or syncope immediately, or delayed by minutes, after rising from a chair or bed. At its most severe, it may result in loss of consciousness and injury, but even milder symptoms may limit medication titration, contribute to fatigue and ‘cognitive fog’ and limit mobility. It occurs as an inherent complication of Parkinson’s disease but also as a side effect of dopamine agonists and levodopa (Table 3).
Urinary symptoms
Urinary frequency, urgency and incontinence occur in up to 70% of patients with LSPD and may contribute to or result from urinary tract infections. Following exclusion of infection and prostatomegaly in men, these authors find low doses of amitriptyline at night useful (eg 10–50 mg at night, starting dose of 5 mg). Low doses remain well tolerated with benefits for nocturia, sialorrhoea, insomnia and pain. Anticholinergics have been associated with long-term risks of cognitive impairment, but this is rarely encountered at low doses. The antimuscarinic solifenacin has been specifically studied in Parkinson’s disease because of its relative affinity for bladder M3 receptors. Desmopressin may be considered for nocturnal polyuria.
Palliative care
Patients with Parkinson’s disease have a high symptom burden with needs similar in intensity to patients with advanced cancer. The prospect of disability often leads to anticipatory grief for patients and families. In those experiencing advancing disease, it is useful to discuss the potential disease-related changes, prognosis and outcomes of medical interventions with the patient and their family to understand patient preferences and philosophy regarding future medical care. As a result of the likelihood of impaired decision-making capacity with disease progression, these conversations are best revisited regularly with an emphasis on maximising quality of life. Pain in Parkinson’s disease is discussed elsewhere.6
Maintaining quality of life in LSPD involves pragmatic decision making, and it is important to explore whether the potential risks of aspiration and falls are outweighed by the significant patient-experienced burden of cessation of all oral intake or confinement to the bed or wheelchair.
When the patient no longer has capacity to engage in these discussions, such decisions fall to the next of kin, who is also often their caregiver. Referral to subspecialty geriatric services, support groups and regional specialist nurses via each state’s Parkinson’s organisation can lend additional supports to families assuming this new role. Such support can and should continue on transition to a residential facility, where outreach services are often available. In these authors’ movement disorders clinic, patients are continually reviewed from residential care in person or via telehealth, and staff are encouraged to maintain contact with the Parkinson’s disease clinical nurse consultants to troubleshoot issues as they arise.
Caregiver burden and quality of life in LSPD
Communication with patients, families and caregivers is crucial, requiring regular briefings about likely changes in symptoms as the condition progresses. Caregiver burden must be recognised and managed to prevent carer burnout and premature institutionalisation of the person with Parkinson’s disease. Realistic expectations need to be communicated. Patients with LSPD become increasingly dependent on family and carers and require assistance with many, or most, activities of daily living. Sixty-five per cent of informal carers report feeling worn down, and that their caring responsibilities are ‘always on [their] mind’, accompanied by feelings of guilt when they are not there.20 Access to the national disability insurance scheme or aged care assessment teams should occur pre-emptively. Sixty-one Parkinson’s disease and movement disorder nurse specialists are employed nationally21 and may provide outreach care and/or advice on a consultation basis through local health districts, industry partners and consumer organisations. Such expert support frequently avoids hospital admissions and optimises community care for patients and their families. Respite care and day centres may ease carer burden. Carers should also be advised to look after their own health; make time for themselves (an often-difficult task); ask for support, including from friends and family; watch for burnout; and understand their limits. With increasing needs, supported living or residential care is ultimately required for many – a difficult decision for patient and carer. Where it is clear that residential care is required, these authors frequently assume responsibility for initiating this conversation, which may help to ease the guilt on carers. Entering residential care can also help to normalise relationships as the carer role is relinquished to staff.
Conclusion
The care of patients with LSPD is challenging. Multiple specialties and allied health professionals may be involved, including neurology, psychiatry, palliative care, gastroenterology, nutrition and dietetics, physiotherapy, speech pathology and social work, with the GP playing a central part. Primary caregiver engagement is vital, as well as access to adequate support services. For those in residential care, regular communication between GP, nursing staff and specialist neurology or gerontology services is advised. Patient comfort, safety and quality of life should remain the focus while providing caregivers with as much support as possible.