Chronic pain is a leading cause of disability and disease burden worldwide.1 Approximately 20% of Australians experience chronic pain.2 They often see their general practitioners (GPs) and are prescribed long-term opioids to manage this.2 This is echoed in North America and Europe, while much of the rest of the world continue to have limited access to opioids.3 Unfortunately, there is strong evidence of significant harms, increasing pain and poorer health outcomes with long-term use of prescription opioids for chronic pain.4–7
GPs find managing prescribed opioid use in patients with chronic pain difficult and conflictual.8 They struggle to introduce other management options.9 They find it difficult to cease opioids commenced by non-GP specialists.10 GPs feel unsupported by pain services, which in the past provided high-dose opioids and expected GPs to continue prescribing these.10 Regulatory processes and risk of censure greatly concern GPs,11 leading to reluctance or complete disengagement from opioid analgesic prescribing.12
Prescription opioid use disorder (pOUD) is not uncommon in patients prescribed opioids for chronic pain.13 Opioid use disorder (OUD) is defined in the Diagnostic and statistical manual for mental disorders, 5th edition (DSM-V) as ‘a cluster of cognitive, behavioural, and physiological symptoms indicating that the individual continues using the substance despite significant substance-related problems.’14 The diagnosis of pOUD is complicated by the exemption of the physiological symptoms of tolerance and withdrawal from the definition.
While not every patient with opioid dependency needs it, opioid agonist treatment (OAT) is a highly evidence-based management option for pOUD that GPs appear reluctant to prescribe.15 In 2019, the National Opioid Pharmacotherapy Statistics Annual Data reported that there were 2844 private prescribers (most of whom were likely to be GPs) providing OAT Australia wide.16 With 36,938 GPs working in Australia,17 this means that fewer than 7% of GPs provide this care. Patients often access OAT care via specialist alcohol and other drug (AOD) services directly.18 Communication, collaboration and formal referral between GPs and specialist AOD services in Australia have been low.19–21 Given the risk of pOUD, it is possible that GPs do not diagnose this in their patients or, if they do, may not offer or refer for management. This may prevent or delay evidence-based management, leading to increased morbidity or mortality.
The aim of this scoping review was to investigate the available literature regarding GPs’ experiences diagnosing and managing pOUD in patients who may have developed this due to long-term opioids prescribed by GPs for chronic pain.
Approach
Review question
Does existing academic literature adequately explain GPs’ knowledge, experience and attitudes towards diagnosing and managing pOUD in patients with chronic pain who may have developed pOUD as a result of long-term opioids prescribed by GPs?
Inclusion criteria
Included studies must have evaluated the experience, attitudes or behaviour of GPs and be original research, reviews or commentaries with a focus on addiction, misuse, extra-medical use, dependence, diversion, aberrant medication-related behaviours and pOUD in patients prescribed opioids for chronic pain.
Exclusion criteria
Studies were excluded if they:
- focused on the experience of GPs who were authorised OAT prescribers outside of the context of chronic pain and pOUD
- focused on risk reduction approaches to opioid prescribing without mention of pOUD
- solely described patient experience.
Participants
General practitioners (or physicians, family or physicians) working in primary care (or general practice or family practice or primary healthcare) were included.
Concept
Studies that evaluated GP experience of pOUD diagnosis and management of patients who may have developed this as a result of prescribed opioid use for chronic pain were included.
Context
This scoping review examined GP experience in English-speaking countries. Studies from all locations in primary care were included. Inpatient or tertiary care studies were excluded. Only English-language literature was included.
Types of sources
This review included all original study designs, commentaries and reviews. In addition to searching peer-reviewed literature, government and department of health websites were searched and conversations with fellow researchers were undertaken to ensure all potentially relevant literature was reviewed.
Methods
A preliminary search was conducted by HW for existing scoping reviews on the topic with no results (September 2020, via JBI Evidence Synthesis, MEDLINE and Embase); therefore, the present review was undertaken. The Joanna Briggs Institute methodology for scoping reviews was used.22 The search strategy aimed to locate both published and unpublished studies, commentaries and reviews. We conducted an initial exploratory search of MEDLINE (Appendix 1) and consulted with subject experts for key articles within the inclusion criteria. The assistance of an experienced database librarian ensured a robust search. Key articles and key indexed terms helped refine the database search. The concepts of opioid, GPs and pOUD were searched, combining keywords, indexed terms and phrases and adapting these for each database.
The following databases were searched: MEDLINE, Embase, PsycINFO (all via OvidSP), Scopus, Emcare, CINAHL and Web of Science from inception to present. A grey literature search was conducted by searching ‘GPs and opioid prescribing’ on government health websites for the USA, Canada, European Union, Australia and New Zealand.
One author (HW) performed the search and collated all identified records into Covidence (www.covidence.org). Duplicates were removed, and titles and abstracts were screened against the inclusion criteria. Two authors (HW and BHR) independently screened the full text documents. Any discrepancies and disagreements that arose were resolved through discussion with the second reviewer (BHR). Additionally, HW searched the reference lists of included articles for further relevant articles, which were included and reviewed by HW and BHR. Given the exploratory nature of this scoping review, the risk of bias and rating of quality of evidence was not assessed for each study; therefore, results cannot be graded. Figure 1 summarises the results of the search.
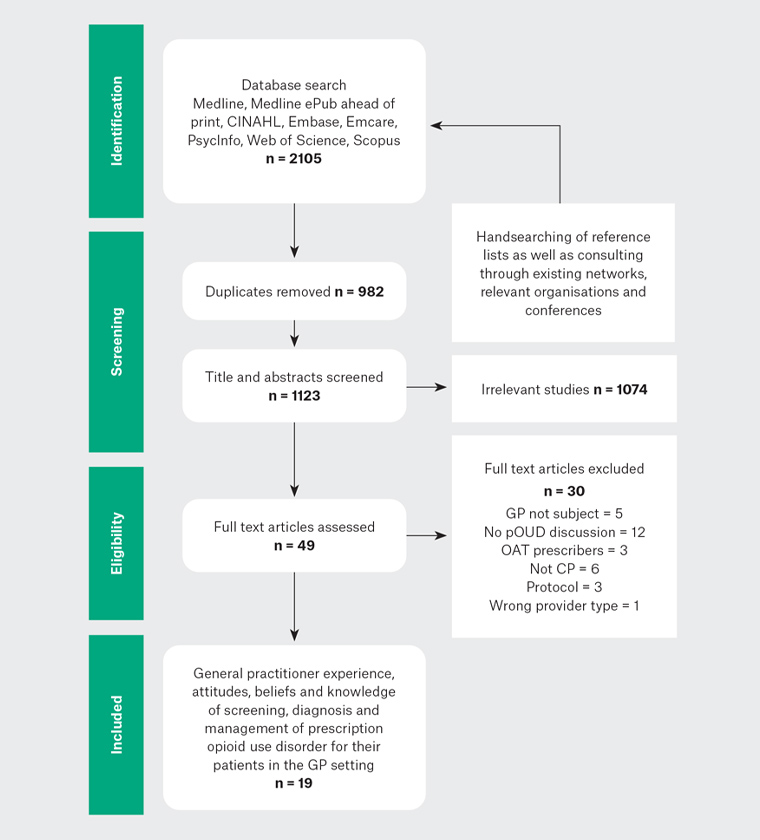
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses database search results
CP, chronic pain; GP, general practitioner; OAT, opioid agonist treatment; pOUD, prescription opioid use disorder
Results
The search yielded a total of 2105 citations. Duplicates were removed, the titles and abstracts of 1123 citations were screened, and irrelevant titles and abstracts were excluded (n = 1074). The remaining 49 publications were read in full, and 30 articles were excluded, leaving 19 citations. A data extraction form was generated from Covidence and used to describe the included studies (Appendix 2).
Analysis was complicated by the studies’ varied aims and methods. The studies used diverse terminology for GPs and included participants with diverse professional roles, such as nurses and pharmacists (Table 1). Some studies included patients as well as practitioners as participants. Primary care structures and environments in the studies’ countries were disparate. For example, one US study setting was described as ‘Veterans Affairs outpatient primary care clinics’,23 while one Canadian study was undertaken in ‘community/urban hospitals, and family care organisations’.24 In addition, the environment in countries with universal primary healthcare (ie Australia, UK, New Zealand and Canada) may have led to a very different experience of healthcare provision when compared with those without uniform healthcare.
| Table 1. Details of studies in this review |
| |
Terms used to describe doctors working in primary care (GPs) |
Other providers included in the studies |
| USA |
Office-based physicians, primary care physicians, family medicine, family practice |
Internal medicine, psychiatrists, paediatricians |
| UK |
GPs, family medicine specialists |
Pharmacists, nurse practitioners, pharmacy technicians, dispenser |
| Australia |
GPs, general practice trainees |
Nil |
| Canada |
Physicians, family physicians |
Nurses |
| New Zealand |
GPs |
Community pharmacists, key experts |
| South Africa |
GPs, specialists in family medicine |
Pain medicine specialists |
| GP, general practitioner |
There were no studies relevant to the research question prior to 2008. Thirty-two per cent of the studies were carried out in the USA, with the remainder in New Zealand (21%), Australia (16%), the UK (11%), Canada (11%) and South Africa (5%). Some studies published multiple articles.20,21,25–29 One study by Kennedy et al is a qualitative review of published studies. It focused on GPs’ ‘opioid prescription experiences’, and some of the articles in our research also form part of the Kennedy study.30
The methods used in the included studies were heterogenous (Appendix 2; Table 2). Just over half the studies were qualitative, and the majority of these were undertaken in the USA and New Zealand. All the included quantitative studies used cross-sectional non-validated surveys that could not describe change over time. The same cross-sectional non-validated survey was used to study over-the-counter codeine issues in the UK and South Africa.31,32 Response rates in the quantitative studies were generally small, with an average response rate of 23% and a range from 1% to 75%. The sample frame is not clear in Bates et al.33 The qualitative articles were most likely to use semi-structured interviews. Other qualitative methods included focus groups, observation and narrative review. Few of the qualitative articles used theory to frame their research.
| Table 2. Country, year and study methods (n = 19) |
| Attributes of studies |
n |
| Year of publication |
2008–10 |
4 |
| 2011–13 |
7 |
| 2014–16 |
4 |
| 2017–19 |
4 |
| Quant, qual, all |
| Study country |
US |
2, 4, 6 |
| UK |
0, 2, 2 |
| Australia |
0, 3, 3 |
| Canada |
2, 0, 2 |
| New Zealand |
3, 1, 4 |
| South Africa |
0, 1, 1 |
| Multiple countries |
1, 0, 1 |
| Qualitative n = 11 |
Semi-structured interviews |
8 |
| Focus groups |
1 |
| Observational and semi-structured interviews |
1 |
| Qualitative review |
1 |
| Quantitative n = 8 |
Cross-sectional survey |
8 |
| quant, qualitative; qual, qualitative |
The studies describe high levels of GP concern regarding the risk of opioid use in chronic pain. The articles use the terms addiction, opioid dependency, inappropriate use, misuse, abuse and diversion. Some use the term ‘aberrant behaviour’ as a marker for addiction.20,25 The term pOUD is only used in one article.34 None of the articles specifically define the terms used. A number of the studies included other psychoactive medicines as well as opioids.27–29,33,35 Two of the articles describe GP experience prescribing OAT.21,25 They do not, however, specifically describe GP experience managing pOUD in patients prescribed opioids for chronic pain. These two articles were included as they are part of larger studies that, like the rest of the articles in this review, focused on the experience of GPs managing patients prescribed opioids for chronic pain. Only four studies (six articles) provide information that spans the issue of interest in this review.20,21,25,26
None of the articles focus specifically on the diagnosis and management of pOUD by GPs in patients prescribed opioids for chronic pain, nor do they discuss this in any detail. They tend to explore either the primary providers’ experience of patients with chronic pain and the negative emotions and impact on providers as they try to avoid pOUD in patients prescribed opioids or their experience and willingness to prescribe OAT generally and not specifically for pOUD. They do not discuss the diagnosis and management of pOUD in patients with chronic pain using opioids who have developed pOUD as a result of primary providers’ prescribing (Figure 2). As a result, only the segments of each article that specifically address diagnosis and management by participants of pOUD in patients prescribed opioids for chronic pain are discussed in this review.
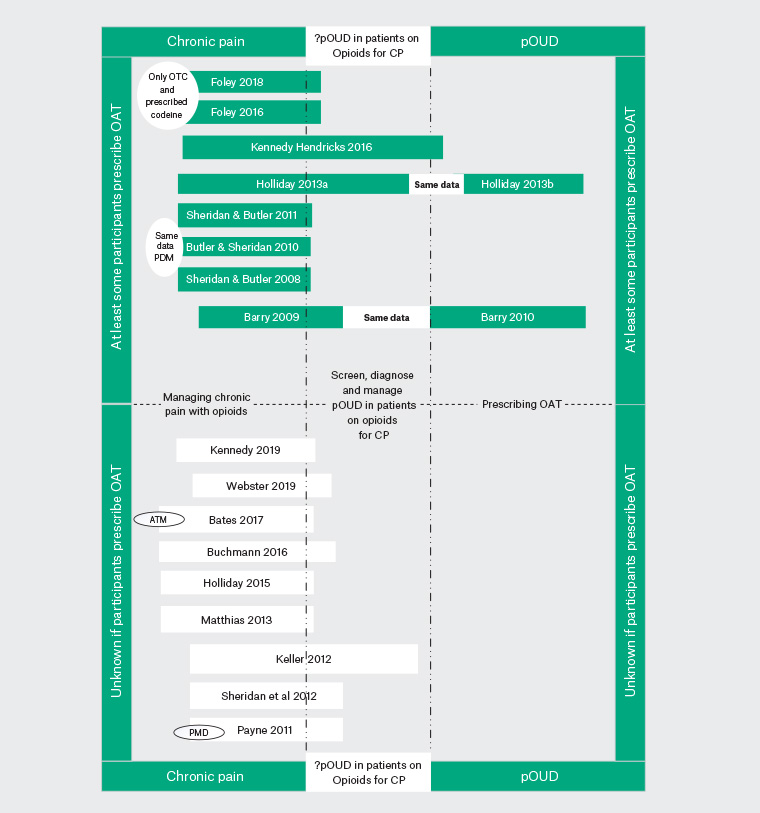
Figure 2. The articles and how they overlap with prescription opioid use disorder in patients prescribed opioids for chronic pain. Click here to enlarge.
ATM, addiction to medicines; CP, chronic pain; OAT, opioid agonist treatment; OTC, over the counter; PDM, prescription drug misuse (includes opioids and other medicines); PMD, psychoactive medication disorders; pOUD, prescription opioid use disorder
Screening and diagnosis of pOUD
Screening and diagnosis of pOUD were haphazard in these studies. Participants often suspected addiction to medicines.33,35 Some reported being confident screening and taking action if they suspected ‘misuse’.20,33–36 Others reported low levels of confidence screening and identifying dependence.23,31,37,38 Participants suggested that risk factors for misuse and abuse were difficult or impossible to characterise with unclear symptoms and subtle nonspecific characteristics, and found clinical ambiguity made decision making difficult and comorbidities made this harder.37 Participants were vague about how they would diagnose pOUD.24 Only 17% reported initiating conversations regarding ‘addiction to medicines’33 and 25% reported refusing to continue prescribing.33 Some participants reported finding it difficult to identify or confirm who was misusing without the patient29,31,32 or other sources informing them first.37,38 Urine drug screens were not often used to aid diagnosis.35 Some reported usually not screening for dependency either at the start of or during opioid treatment for chronic pain.25 It was not uncommon to continue to prescribe opioids for chronic pain in patients with opioid dependency.20,36 Sheridan noted that participants stated they were confident recognising prescription drug misuse in her 2012 quantitative survey.35,39 This strongly contrasted low levels of confidence found in her earlier qualitative articles.27–29 She suggests that participants may have answered questions in ways that were ‘socially desirable’.35
Participants’ perceptions of patients were important barriers. Younger patients were seen as ‘abusers’ and less likely to be offered any treatment while older patients with ‘genuine’ pain were thought to be ‘overusers’.27 The dichotomous worthy–unworthy attitudes to the suffering, genuine patient with pain versus manipulative drug users, trying to get opioids to sell or to get high, led to distorted perceptions of risk. This suggests that patients who are at risk of harm, when seen to have ‘genuine pain’ or be ‘drug seeking’ may not be diagnosed or offered treatment.27 There was seen to be a fine line between pain control and abuse that made conversations difficult.24 Participants struggled with issues of patient trust and deception.38 They were aware of the complexities of many patients’ presentations and struggled with skills and resources to manage these.24
Management of pOUD
OAT prescribing by GPs was uncommon. It is likely that 10 of the articles had participants who had training and expertise in prescribing OAT. Eight of the articles noted participants’ OAT prescribing status,20,21,25,27,29,34,40 with a range from 13%34 to 53%.29 Two other articles reported training in ‘substance misuse’, and it is likely that this involved OAT prescribing.31,32 Barriers to undertaking OAT prescribing included: inadequate financial reward, lack of confidence, lack of specialist support, stigma, low prevalence of pOUD, colleague objections and inadequate training.21,25,26,30 These barriers were lower for older GPs and current OAT prescribers.25 Participants showed variable levels of interest managing pOUD,26 with 9% of participants stating they would consider providing OAT for a patient with codeine dependence31 and 32% suggesting they were comfortable prescribing OAT but did not do this often.38
Patients with opioid issues were likely to be referred to addiction services.21,31,32,35 Forty-three per cent of participants reported at least one referral in the past month,31 while the level of referral of a patient to a ‘specialist’ varied from 27%31 to 43%32 to 68%.35
Discussion
GP experience of diagnosis and management of pOUD in patients prescribed opioids for chronic pain is strangely absent from the literature. The articles in this review cover the complex issues GPs face managing patients with chronic pain with prescription opioids. The negative emotions, complexity of presentations and lack of systematic screening for pOUD are described. Little appears to have changed over time. The articles do not fully cover the issues GPs face when they diagnose pOUD in patients with chronic pain or their experience managing this, with or without OAT.
We have seen huge changes in the approach to opioid use in the management of chronic pain over the past 20 years. It has moved from an opiophilic to opiophobic approach that does not always account for the unique presentation of a patient. This has risk of harm itself, which needs to be acknowledged.41
Similar to many studies, the articles in this review use terms such as opioid abuse, diversion, inappropriate use, aberrant behaviours, misuse, dependence and addiction. This is despite the fact that over time the DSM and International Classification of Disease have changed their nomenclature. DSM-III, first published in 1980, was the first to acknowledge that substance abuse and substance dependence were primary conditions. DSM-V, published in 2007, moved away from the terms ‘abuse’ and ‘dependence’ to ‘substance use disorder’, which describes a continuum of signs and symptoms. This change in nomenclature is not reflected in the literature. There is no doubt that pOUD diagnosis in patients with chronic pain is difficult. This diagnosis creates controversy even among pain and addiction specialists, with 86% agreeing that more work was needed ‘to better define diagnostic criteria of dependence and addiction related to prescribed medications for therapeutic reasons’.42
While not everyone who develops risky opioid use has dependence, and not everyone who develops dependence needs long-term OAT, it is a highly effective, evidence-based treatment for pOUD.43 It ‘remains out of reach to those to need it, in all the countries studied. Even in Australia, patients wait for months to years to access treatment, with patient numbers in OAT treatment stable at 20 per 10,000 people since 201044 despite increasing rates of opioid dependency. Few patients with concurrent chronic pain and pOUD report lifetime engagement in OAT.45
GPs continue to experience difficult consultations and negative emotions associated with these consultations. This experience is linked to negative attitudes and stigma. Stigma and the human tendency to label and create ‘outgroups’ leads to status loss and discrimination for the individual and stigmatised groups.46 Interventions to change GP attitudes may be useful but may not be enough to ensure GPs undertake interventions.47
The environmental settings and health systems in many of the included articles are not comparable to the Australian general practice setting. The Australian articles are limited by scope and research design. There are no Australian articles that describe the GP experience of this challenging area in any depth. This paucity of research suggests that qualitative research will help to understand the depth of the barriers faced by GPs in diagnosing and managing pOUD in their patients prescribed opioids for chronic pain. Using a theoretical basis for studies that look at the complex dimensions of this issue may be useful. Research must address the attitudes, constraints, social and systemic controls that limit successful engagement. This may assist in developing and piloting interventions for GPs to assist patients who have developed pOUD as a result of opioids prescribed for chronic pain.
Limitations
This review is limited to English-language articles. As a scoping review, it does not seek to assess the quality of these articles. More than half the studies have some participants who were already prescribing OAT. As such, they may be more open to diagnosing and managing pOUD. The diverse range of professions and settings also limits generalisability of the results to the Australian general practice setting.
Conclusion
There is limited research focused on the experience of GPs diagnosing and managing pOUD in patients prescribed opioids for chronic pain. It is possible that negative emotions, previous adverse experiences, lack of knowledge, low confidence, complex presentations, absence of systemic screening and diagnostic confusion are instrumental in shaping this experience. This may contribute to decreased access, delayed treatment uptake and poorer patient outcomes. Research is required to investigate the specific contextual factors and facilitators needed for GPs to engage in diagnosis and management of pOUD in patients prescribed opioids for chronic pain. This may assist the development of future programs, research and policies, leading to greater diagnosis and effective patient management for this important condition.