Imaging algorithms for coronary artery disease (CAD) screening differ significantly from diagnostic studies evaluating cardiac symptoms including chest pain. Cardiac imaging is applicable to a wide variety of pathologies and conditions. For the purposes of discussion, CAD and cardiomyopathy are the entities predominantly evaluated.
Imaging related to possible cardiac symptoms can be broadly grouped into functional and anatomical imaging groups with a common end point of diagnosis or exclusion of significant CAD. Invasive or conventional coronary angiography remains the gold standard for diagnosis of CAD with the ability to define anatomy of stenoses and to assess the functional flow reserve (FFR) or true haemodynamic significance of visualised plaque.
X-ray
Plain chest X-ray imaging is a very useful starting point that is readily available and exposes patients to a low radiation dose. It allows for exclusion of many causes of chest pain and dyspnoea, including pneumothorax, pneumonia, pleural effusion and mass lesion. The presence of cardiomegaly can be confirmed and suggests a pathway for further imaging evaluation.
Coronary artery calcium
Coronary artery calcium (CAC) or coronary calcium score (CCS) scoring has become a widely used non-invasive method to stratify risk in asymptomatic patients in association with standard risk factors including diabetes, hypertension, hyperlipidaemia, family history and smoking.1 The CAC score stratifies patients on the basis of likelihood of having a coronary event in the next five years (ie low-, intermediate- and high-risk groups). CAC imaging is widely available with a low radiation dose and provides data of very high specificity, nearing 100% (Figure 1).2
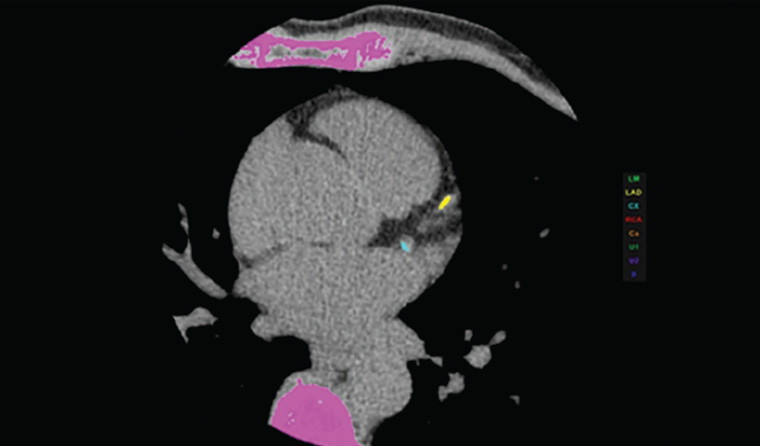
Figure 1. Calcium depicted in colour on a coronary calcium score computed tomography scan
CAC has emerged as a determinant study for stratification of CAD burden and is best applied in the intermediate-risk asymptomatic adult population. Calcium scores >100 or >75th percentile suggest more aggressive medical therapy is required. In the highest risk group, with a score >400, further investigation is indicated initially with computed tomography coronary angiography (CTCA).
Single-photon emission computed tomography myocardial perfusion scan
Single-photon emission computed tomography (SPECT) myocardial perfusion scan (MPS) is a validated, longstanding technique (Figure 2). Sensitivity and normalcy of SPECT MPS in diagnosing CAD are 85–90% and 89%, respectively. A normal study is a prognostic indicator of a <1% annual risk of a coronary event, while an abnormal MPS indicates a seven-fold likelihood of acute myocardial infarction within 10 years when compared with the normal group.3 Radiation doses have reduced significantly in recent years, as for all imaging associated with ionising radiation.
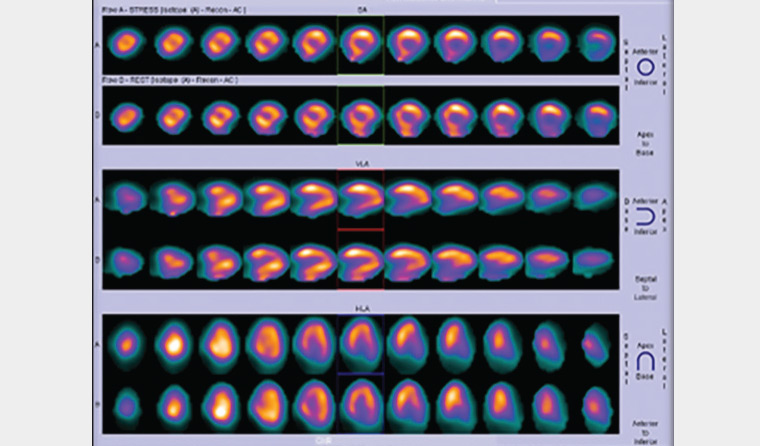
Figure 2. Myocardial perfusion scan showing fixed ischaemic mural changes
At this stage, functional quantitative assessment with SPECT MPS is very useful. If this study confirms reversible ischaemia, then referral for consideration of invasive angiography and stent placement is appropriate. Indeterminate findings warrant CTCA rather than invasive angiography because of its low risk of morbidity and ease of access. The procedure is very well tolerated by patients. In this author’s experience, the most common comment following CTCA is, ‘What was all the fuss about? Is that all there is to it? Why was I so worried?’
Stress echocardiography
Stress echocardiography is a useful technique for stratifying patients and is widely available. In some centres this modality is used to direct patients to MPS if the study is equivocal and to CTCA if the study is abnormal. Expertise in performing and interpreting stress echocardiography is becoming more widespread. Transoesophageal echocardiography is useful for more detailed assessment of valvular and aortic structures. It is used as a problem-solving study rather than a routine investigation.
Echocardiography can demonstrate chamber size, regional wall motility abnormalities, pericardial disease and functionality of cardiac valves. It is a very useful tool for suggesting the presence of a cardiomyopathy. Further assessment with stress echocardiography provides detail of ischaemia-induced mural changes.
MPS and stress echocardiography are modalities that allow non-invasive assessment of segmental ischaemia.
Computed tomography
Cardiac positron emission tomography–computed tomography (PET-CT) is a newer modality with further dose reduction, higher resolution and ability to quantify perfusion (Figure 3). However, it is unlikely to become widely used as a result of cost and PET-CT scanner time availability. This modality may become increasingly available in the future.
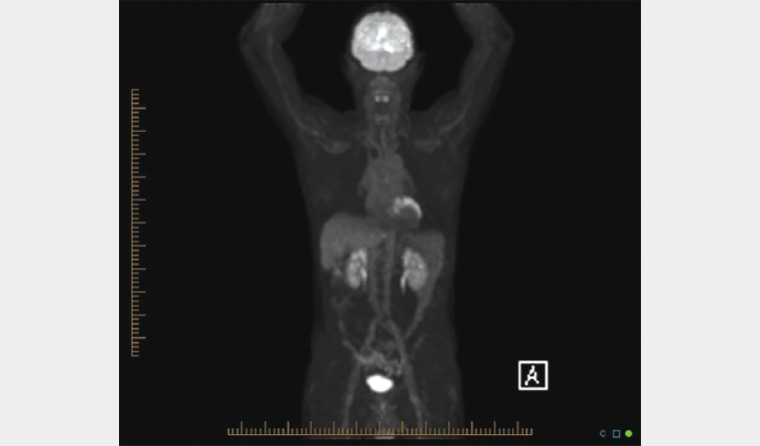
Figure 3. Positron emission tomography–computed tomography scan showing chronic myocardial ischaemia
CTCA (Figure 4) has become a mainstay of cardiac imaging over the past 12–15 years and has evolved into having a triage role with utilisation across general and specialist practices.
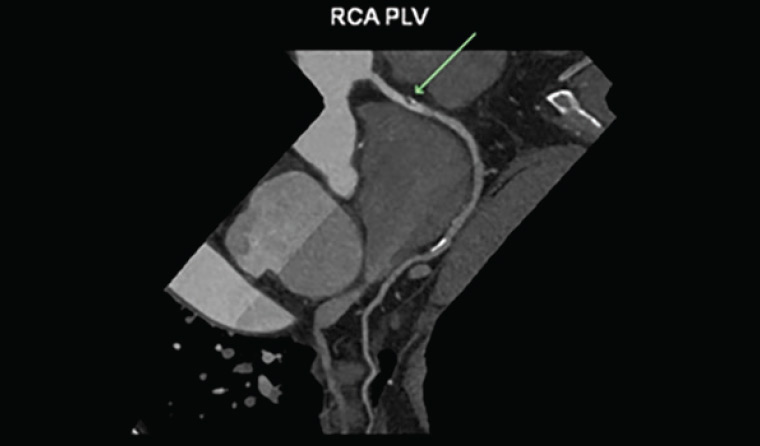
Figure 4. Soft mild <50% right coronary artery stenosis on computed tomography coronary angiogram (arrow)
A CTCA that is negative (or normal) has a negative predictive rate for future cardiac events of near 100%.4 However, non-calcified plaque that is not identified on CAC studies can be well demonstrated and places the patient into a different risk group, which may indicate the need for disease-modifying therapies including statin treatment.
CTCA with grossly elevated plaque burden may be non-diagnostic because of blooming artifact related to the calcium load (Figure 5). These cases should be referred for invasive or functional imaging such as MPS or stress echocardiography if these have not been performed already.
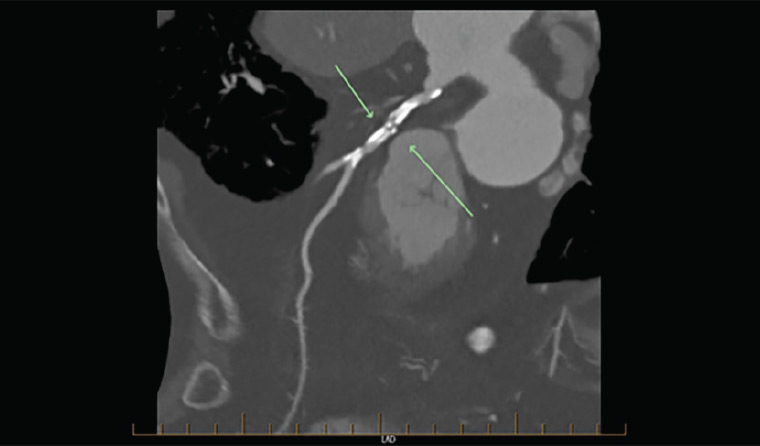
Figure 5. Gross coronary artery calcification obscuring detail (arrows)
CTCA reporting stratifies patients into groups on the basis of the plaque burden and number of vessels involved.5 The group with low-to-moderate plaque burden can be triaged for medical therapy or referred for invasive angiography with a view to assess suitability for stent placement or coronary artery interventions including bypass graft placement or intraluminal plaque treatment.
Currently functional assessment of stenoses is limited, although research into FFR is progressing, and in the future it should be possible to more accurately quantify the degree of haemodynamic compromise distal to the plaque. Dual-energy CT is not currently widely available but will contribute significantly to resolving this issue.6,7
CTCA is also a significant non-invasive technique for assessment of bypass graft and intraluminal stent patency (Figure 6). Stent patency and restenosis estimation is patient dependent, and accuracy is limited. This is an area that will benefit from further advances in CT technology. Anastomotic graft stenoses may also be evaluated.
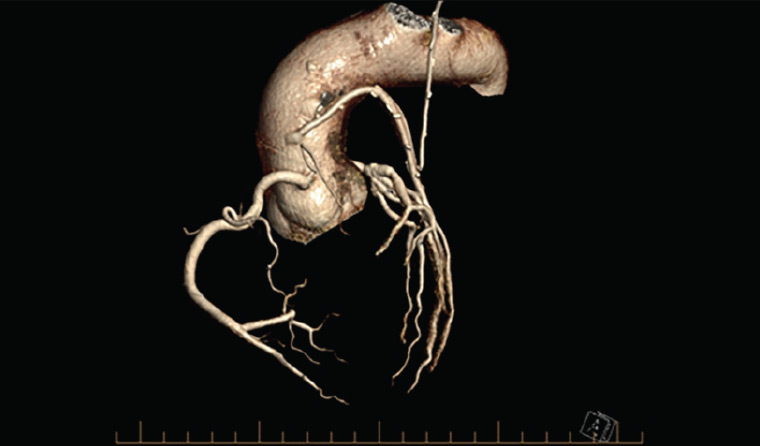
Figure 6. Three-dimensional graft study generated from computed tomography coronary angiogram
Coronary artery aneurysms, vasculitis and anomalous vessel origins are also well demonstrated on CTCA. Incidental findings may include aortic dissection, aneurysm and proximal pulmonary emboli. Intraventricular lesions, such as cardiac tumours and blood clots, can be readily demonstrated.
Depending on the results obtained, it may be appropriate to proceed without further intervention; however, patients who would benefit from bypass surgery or stent placement will have been identified.
Unfortunately, the ability to assess the haemodynamic significance of a coronary artery stenosis shown on CTCA is not yet widely available. Dual-energy CT shows great promise in tissue characterisation and myocardial perfusion, allowing for significant functional assessment.
Magnetic resonance imaging
Cardiac magnetic resonance imaging (MRI) has a growing role in evaluating structure, function and myocardial tissue characterisation (Figure 7). Ejection fractions, ventricular volumes and myocardial mass can all be calculated. Viability of myocardium with the presence of oedema or fibrosis is readily visualised.8,9
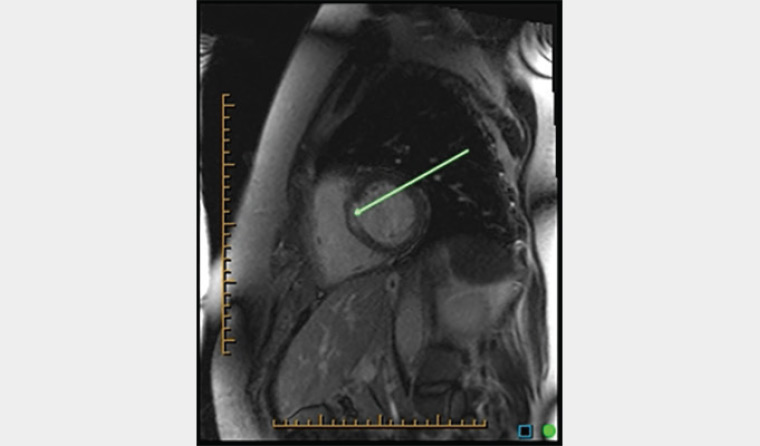
Figure 7. Magnetic resonance imaging depicting subepicardial ischaemic changes
Hypertrophic cardiomyopathy is one of the most common forms of cardiomyopathy and is usually first detected on echocardiography.
However, MRI and cardiac CT (when MRI is contraindicated) can demonstrate morphological left ventricular wall changes with dynamic imaging and, unlike echocardiography, can accurately quantify myocardial fibrosis/scarring (Figure 8).10
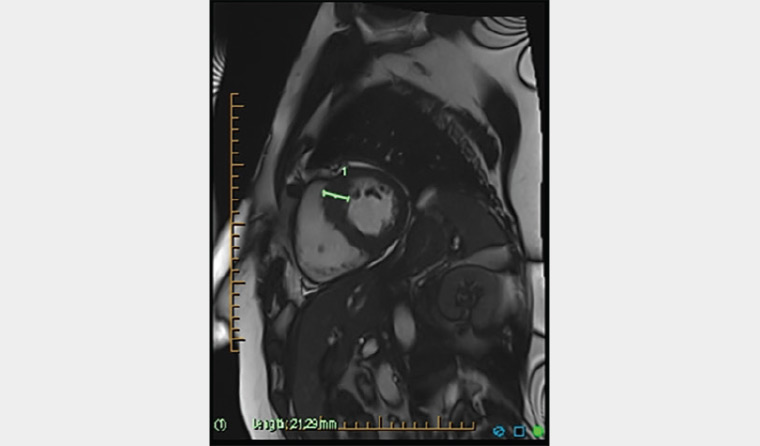
Figure 8. Sagittal cardiac magnetic resonance imaging of hypertrophied left ventricular wall
Cardiac MRI is predominantly a tertiary imaging study. The role of MRI is presently not well defined in non-tertiary centres. It is most promising for assessment of myocardial viability post infarction or regional wall motion abnormalities, and exclusion of infiltrating entities such as sarcoid and amyloidosis. MRI assessment of coronary arteries is not comparable with CTCA at this stage, but further advances will undoubtedly lead to greater accuracy.
The dataset volumes generated in cardiac MRI and CT can be overwhelming, with >10,000 images not uncommon. Artificial intelligence and computer-assisted reporting is increasingly sophisticated and will alleviate some of the burden of reporting these studies.
Invasive angiography
Invasive angiography should be reserved for problem solving if other studies cannot adequately characterise abnormal findings – for example, in the setting of grossly elevated CCS and suboptimal detail on CTCA. CTCA requires only venous access for contrast injection, as opposed to arterial puncture and catheterisation with its attendant risks involved in invasive conventional angiography. The other major role of invasive angiography is providing intraluminal access for percutaneous stent insertion.
Conclusion
Cardiac imaging has evolved dramatically over the past 20 years, and the roles of functional and anatomical imaging modalities are converging. The next most significant step may possibly be the ability to routinely assess the haemodynamic significance of stenoses of coronary arteries with non-invasive CTCA.