Viral illnesses are the most common cause of myocarditis, an inflammation of the myocardium. The aim of this article is to provide an overview of myocarditis, particularly in the context of SARS-CoV-2 infection and mRNA vaccines.
Cardiac manifestation of SARS-CoV-2 infection is associated with a wide range of presentations (Table 1) from asymptomatic biomarker elevation to serious ventricular or supraventricular arrhythmias, left ventricular dysfunction and heart failure.
Patients hospitalised with COVID-19 frequently have elevated levels of cardiac biomarkers, such as natriuretic peptides, troponins, myoglobin, C-reactive protein, interleukins and ferritin, which is the result of myocardial injury. The possible mechanisms of cardiovascular injury include direct viral effect on myocytes, angiotensin converting enzyme-2 receptor–mediated injury, microvascular dysfunction, thrombosis and an excessive immune inflammatory response such as a cytokine storm.1
Myocardial injury, defined as elevated high-sensitivity cardiac troponin (hs-cTn), is commonly seen in patients hospitalised with COVID-19 and is associated with an adverse prognosis.2 Weber et al recently showed that 62.3% of patients hospitalised with COVID-19 had a positive hs-cTn (≥14 ng/L) during the index hospitalisation, which was associated with increased mortality (hazard ratio: 13.76; 95% confidence interval [CI]: 1.85, 102.3; P = 0.01). Among patients who survived their index hospitalisation, the incremental mortality through 12 months was low, even among patients who were troponin positive.
Myocarditis
Although viruses are the most common cause of acute myocarditis, it can result from a wide spectrum of other infectious pathogens, including bacteria, chlamydia, rickettsia and protozoans, as well as medications, toxic substances and hypersensitivity reactions. Myocarditis also has been reported following both SARS-CoV-2 infection and post mRNA vaccine (Pfizer-BioNTech and Moderna).
The incidence and severity of myocarditis is greater in men than women. Studies in animal models of myocarditis show that testosterone drives the inflammation and susceptibility to develop dilated cardiomyopathy.3
The prognosis of myocarditis varies widely by cause. Patients who recover may later develop dilated cardiomyopathy and heart failure. In up to 30% of cases, biopsy-proven myocarditis can progress to dilated cardiomyopathy and is associated with poor prognosis.4
Consensus definition
According to the consensus paper5 of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases, myocarditis is defined by an inflammation of the myocardium diagnosed by established histological, immunological and immunohistochemical criteria.
Myopericarditis is acute pericarditis with elevated troponin but without left ventricular systolic dysfunction. Myocarditis is an important cause of chronic heart failure and dilated cardiomyopathy.
Pathogenesis
The current understanding of the pathogenesis of myocarditis is based on animal models. In these models, the progression from acute injury to dilated cardiomyopathy may be simplified into a three-stage process:6
- Phase 1: cardiac injury and activation of the innate immune response.
- Phase 2: acute myocarditis involving components of the innate and acquired immune response.
- Phase 3: recovery in resistant individuals versus progression to dilated cardiomyopathy in susceptible individuals.
Acute injury leads to myocyte damage, exposure of intracellular antigens such as myosin, and activation of the innate immune system. Over weeks, specific immunity that is mediated by T lymphocytes and antibodies directed against pathogens and similar endogenous cardiac epitopes causes robust inflammation. In most patients, the pathogen is cleared, and the immune reaction is downregulated with minimum sequelae. However, in other patients, the virus is not cleared and causes continued myocyte damage due to persistent specific inflammation against the endogenous antigens.
The mechanism of vaccine-induced myocarditis is not clear7 but may be related to the active component of the vaccine, the mRNA sequence that codes for the spike protein or the immune response that follows vaccination.
Clinical manifestation and evaluation
In myocarditis, the inflammation can be focal or diffuse, and the clinical presentation can vary. Patients with acute myocarditis can present with non-specific symptoms from chest pain, dyspnoea and palpitations that resolve spontaneously, to asymptomatic biomarkers elevation and electrocardiography (ECG) changes mimicking acute coronary syndrome, cardiogenic shock and sudden death. The non-specific symptoms typically precede the cardiovascular symptoms by a few days to weeks. As a result of variable clinical presentation, myocarditis needs to be considered in the differential diagnosis of patients presenting with such symptoms.
Fulminant myocarditis – a rare, sudden and severe cardiac inflammation – is one of the main causes of cardiogenic shock in young adults.
In a patient with suspected myocarditis, more common causes of cardiovascular disease, such as acute myocardial infarction and valvular heart disease, need to be excluded. Chest pain in myocarditis can result from associated pericarditis or, at times, from coronary artery spasm.
Myocarditis and pericarditis need to be considered as a differential, especially in adolescents or young adults presenting with acute chest pain, shortness of breath or palpitations.
Routine ECG is typically abnormal in patients with myocarditis, but none of these abnormalities are specific enough to establish the diagnosis. Common ECG findings include ST segment elevation in 44.8%, sinus tachycardia in 11.5%, T-wave inversion in 7.3% and ST-segment depression in 5.2% of patients.8
Echocardiography findings in COVID-19-related myocarditis include reduced ejection fraction with both global and regional hypokinesia. An increase in wall thickness and pericardial effusion can be found in 6.9% of cases.8
The US Centers for Disease Control and Prevention (CDC) recommends9 initial evaluation of patients with suspected myocarditis/pericarditis include an ECG, troponin level and the inflammatory marker C-reactive protein. In the setting of a normal ECG, troponin and C-reactive protein, myocarditis or pericarditis are unlikely.
For suspected cases, it is important to consider referral to cardiology to confirm the diagnosis and for assistance with cardiac evaluation and management. Cardiac magnetic resonance imaging (MRI) is a preferred non-invasive test for confirmation of acute myocarditis without the risk of biopsy.10,11 A combination of T2-weighted MRI and post-gadolinium early and late T1-weighted MRI provides the best sensitivity (67%) and specificity (91%) for diagnosis.10 MRI findings in post-vaccination myocarditis frequently shows typical late gadolinium enhancement. Endomyocardial biopsy may be performed in selected cases if the diagnosis remains uncertain.
Physical activity should be restricted during the acute phase of myocarditis and for at least six months thereafter. All patients with myocarditis should be followed up with clinical assessment, ECG and echocardiography.5
COVID-19, mRNA vaccines and myocarditis
Cardiac complications of COVID-19 and mRNA vaccines may have a broad spectrum of clinical presentations (Table 1).
| Table 1. Cardiovascular manifestations of SARS-CoV-2 infection |
| Myocardial injury |
- Elevated high-sensitivity cardiac troponin is frequently seen in patients hospitalised with COVID-19 and is associated with an adverse prognosis.
|
| Myocarditis/pericarditis |
- Most patients present with cough and fever followed by dyspnoea and chest pain. Patients usually have elevated troponin and abnormal electrocardiography and C-reactive protein levels.
- COVID-19-associated myocarditis is most common among children aged <16 years and adults aged ≥75 years.
- Post-vaccine myocarditis has been reported, primarily in younger males several days following a second mRNA vaccine, whereas pericarditis affects older patients after either the first or second dose. The risk seems to be higher with Moderna than with Pfizer vaccines.
- Most cases are mild, and patients recover completely.
|
| Arrhythmias |
- The most common arrhythmia in the acute infectious period of COVID-19 is sinus tachycardia.
- The most common pathological arrhythmia is atrial fibrillation that is associated with a poor prognosis.
- Ventricular tachycardia, ventricular fibrillation and atrioventricular block are rare.
|
| Left ventricular dysfunction/heart failure |
- Focal or global left ventricular dysfunction, stress cardiomyopathy, acute myocardial infarction and heart failure have been reported.
|
| Multisystem inflammatory syndrome in adults (MIS-A) |
- MIS-A is a rare condition of delayed immunological response to SARS-CoV-2 infection in adults with hyperinflammation. Clinicians should consider a diagnosis of MIS-A among patients with hyperinflammatory illness and severe extrapulmonary multiorgan dysfunction, particularly cardiovascular manifestations including myocarditis, reduced ejection fraction, arterial thrombosis, deep venous thrombosis or pulmonary embolism16 occurring within 2–5 weeks of antecedent COVID-19 infection.
|
Based on US hospital observational data,12 the risk for myocarditis among patients with COVID-19 was nearly 16 times as high as the risk among patients without COVID-19, with the association being most pronounced among children aged <16 years and adults aged ≥75 years.
In a study of SARS-CoV-2 infection and myocarditis published by Jaiswal et al, the majority of patients presented with a cough (61.9%) followed by fever (60.4%), shortness of breath (53.2%) and chest pain (43.9%).8 Inflammatory markers were elevated in 97.8% patients, whereas cardiac biomarkers were elevated in 94.8% of the included patients.
Myocarditis has also been reported primarily in younger males within a median of 3.5 days following administration of a second dosage of mRNA vaccine, whereas post-vaccination pericarditis affects relatively older patients, following either after the first or second dosage of mRNA vaccine.13
A 2021 study by Barda et al14 reported that mRNA COVID-19 vaccination was associated with an elevated risk of myocarditis (risk ratio [RR]: 3.24; 95% CI: 1.55, 12.44); in the same study, a separate analysis showed that SARS-CoV-2 infection was a strong risk factor for myocarditis (RR: 18.28; 95% CI: 3.95, 25.12).
One of the largest population-based studies,15 involving more than 23 million people across the Scandinavian countries, confirmed the increased risk of myocarditis after COVID-19 vaccination. The risk seems to be higher with Moderna than with Pfizer vaccines (4–7 excess cases of myocarditis per 100,00 individuals after second dose of Pfizer vaccine and 9–28 excess cases after second dose of Moderna vaccines). The study may suggest that for young men between 16 and 24 years of age, the risk of myocarditis after vaccination with either the Pfizer or Moderna vaccines is higher than the risk of myocarditis after SARS-CoV-2 infection.
Case study: Patient 1
Kayla, aged 18 years, presented to her general practitioner (GP) after waking up with ‘terrible chest pain’ following two days of a headache and runny nose. She did not have a cough, fever, myalgia or gastrointestinal symptoms. She was otherwise well and had received a second dose of a COVID-19 mRNA vaccine one week prior. Her home rapid antigen test was negative.
Her ECG (Figure 1) showed sinus rhythm with diffuse ST segment elevation in most leads, except in lead aVR with PR segment depression, when she was referred to the local hospital emergency department.
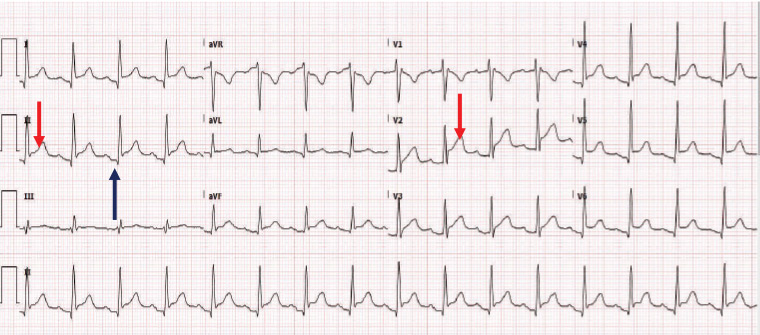
Figure 1. Electrocardiography shows sinus rhythm with diffuse ST elevation (red arrow) in most leads, except in aVR with PR depression (black arrow) when the patient was referred to the local hospital emergency department.
Blood tests in the hospital emergency revealed: white blood cells 8.8 × 109/L (reference range 4.5–11.0 × 109/L), neutrophils 5.44 × 109/L (reference range 2.0–7.0 × 109/L), lymphocytes 1.91 × 109/L (reference range 1.0–3.0 × 109/L), C-reactive protein 103 mg/L (reference range <3 mg/L) and troponin 6861 ng/L (reference range <10 ng/L).
Echocardiography showed normal left ventricular function and an ejection fraction of 67% without any regional wall motion abnormalities.
Kayla made a quick uneventful recovery.
Case study: Patient 2
John, aged 52 years, lived at home with his wife, who tested positive for COVID-19 six days ago. He had an urgent tele-consult with his GP because of lethargy, dry cough and breathlessness for the past two days. He reported that the previous night he had breathing difficulties while lying flat, so he slept on a couch and developed mild peripheral oedema. The GP arranged urgent transfer for John to the nearest emergency department.
John was found to be afebrile and had an oxygen saturation of 84% on room air when he was assessed in the hospital. His lymphocytes count was 0.91 × 109/L and C-reactive protein was 164 mg/L; all other blood test results were unremarkable. A computed tomography (CT) scan of the chest (Figure 2) revealed bi-basal reticular lung markings and opacities, mostly in the periphery. His COVID-19 polymerase chain reaction test was positive.
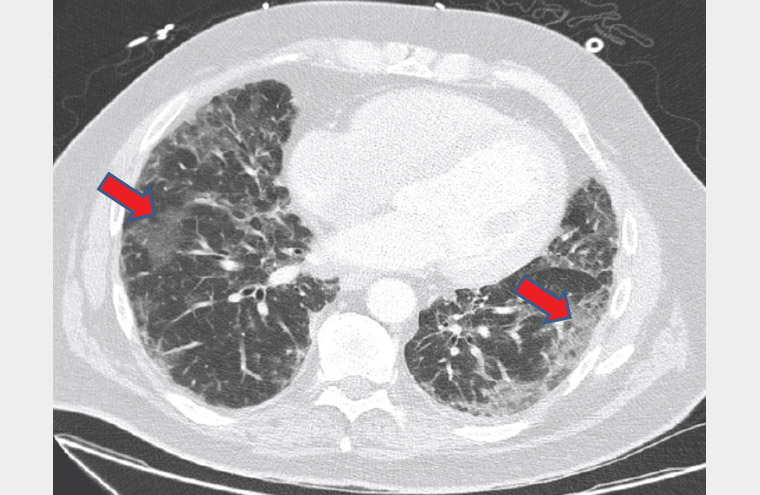
Figure 2. A computed tomography scan of the chest revealing bibasal reticular lung markings and opacities (red arrows)
His ECG (Figure 3) showed diffuse T-wave inversion, and the echocardiography revealed severe global left ventricular dysfunction, consistent with cardiomyopathy.
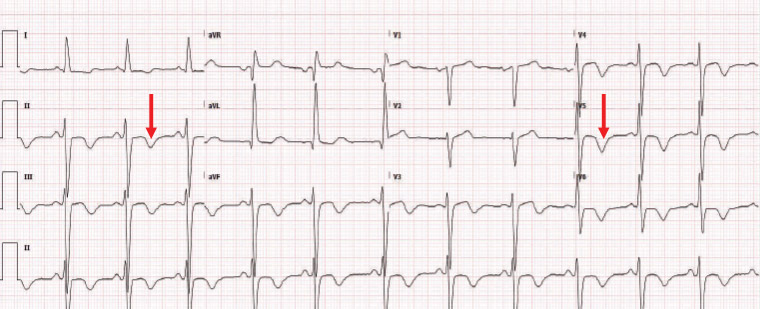
Figure 3. Electrocardiography showing diffuse T-wave inversion (red arrow), and the echocardiography reveals severe global left ventricular dysfunction, consistent with cardiomyopathy.
John had CT coronary angiography that showed no significant coronary artery disease and a cardiac MRI that showed changes consistent with myocarditis. He had a protracted admission in the intensive care unit (ICU) for COVID-19-related pneumonia and myocarditis, from which he slowly recovered.
While in the ICU, he developed atrial fibrillation when he was appropriately anticoagulated. For dilated cardiomyopathy, he received guideline-directed treatment including an angiotensin converting enzyme inhibitor, β-blocker, aldosterone antagonist, sodium–glucose co-transporter-2 inhibitor and diuretics.
Eight weeks after the initial diagnosis, John was reviewed by his GP. He continued to have limited exercise tolerance and low blood pressure, which made it difficult to up-titrate his medications.
Conclusion
Myocarditis, a rare complication, has been reported with both SARS-CoV-2 infection as well as with mRNA vaccines. Most cases are mild, and patients recover completely. COVID-19 vaccine prevents not only severe disease and hospitalisation but also other serious complications and death. This risk of vaccine-induced myocarditis should be balanced against the benefits of protecting against severe COVID-19 disease, particularly in young males.
Key points
- Initial evaluation of patients with suspected myocarditis/pericarditis include an ECG, troponin level and the inflammatory marker C-reactive protein. In the setting of a normal ECG, troponin and C-reactive protein, myocarditis or pericarditis are unlikely.
- For suspected cases, it is important to consider referral to cardiology to confirm the diagnosis and for assistance with cardiac evaluation and management.
- COVID-19 vaccines prevent not only severe disease and hospitalisation but also other serious complications and death. This risk of vaccine-induced myocarditis should be balanced against the benefits of protecting against severe COVID-19 disease, particularly in young males.
- Physical activity should be restricted during the acute phase of myocarditis and for at least six months thereafter. All patients with myocarditis should be followed with clinical assessment, ECG and echocardiography.