Pulmonary embolism (PE) is still the third most common cause of death from cardiovascular disease despite improvements in diagnosis and acute management. PE should be suspected in all patients who present with new or worsening dyspnoea, chest pain or hypotension without alternative cause. Diagnosis relies on clinical pre-test probability, D-dimer testing and objective radiological imaging. While much of the diagnosis and initial management of PE takes place within the acute hospital setting, an understanding of PE by general practitioners (GPs) remains important – in initial diagnostic suspicion, a trend towards community-based management and the need for GP shared care in the longer-term management and monitoring of anticoagulation. Long-term complications include chronic thromboembolic pulmonary hypertension (CTEPH), although this is rare.1
The aim of this article is to describe the epidemiology, clinical presentation, diagnosis, management and outcomes of PE.
Epidemiology
Venous thromboembolism (VTE), which includes both deep venous thrombosis (DVT) and PE, has an incidence of approximately 1–1.5 per 1000 population, which rises with increasing age.2–4 Incidence is increasing, potentially because of improved awareness and more sensitive diagnostic techniques, as well as the ageing population and improved life expectancies for people with comorbidities that increase the risk of VTE, such as cancer.5,6 PE is associated with significant mortality risks, with a reported 30-day mortality rate of 6.4% and one-year mortality rate of 21.6%, contributed to by underlying comorbidities.2,7 Thromboembolism remains the most common direct cause of death in pregnancy in Australia.8 Despite increasing overall incidence, mortality from PE is decreasing, likely as a result of increased use of more effective therapies and interventions.9
Clinical presentation
GPs are often the first port of call for patients who have symptoms of PE. Patients may present with dyspnoea and/or chest pain that is either acute or subacute (days-to-weeks) in onset.10 Other symptoms include haemoptysis, palpitations and pre-syncope or syncope.11 Symptoms at presentation often vary depending on thrombus location, with peripheral emboli causing pleuritic chest pain and/or haemoptysis while larger, central emboli often present with isolated dyspnoea. Presentation with haemodynamic instability or syncope is rare and indicates central or extensive PE.5,11 Lower limb oedema, pain and erythema may indicate concomitant DVT.
Signs of PE are nonspecific and include tachypnoea, tachycardia, hypotension and signs of right ventricular failure. The threshold for further investigation should be low because of the nonspecific nature of symptoms and signs and because the consequences of a missed diagnosis can be dire.
Risk factors for VTE are shown in Table 1.
| Table 1. Hereditary and acquired risk factors for venous thromboembolism (VTE)37–40 |
| |
Risk factor |
Risk |
| Hereditary*11,37 |
Antithrombin deficiency |
RR 5–10 |
| Protein C deficiency |
RR 4–6.5 |
| Protein S deficiency |
RR 1–10 |
| Heterozygous factor V Leiden |
RR 3–5 |
| Heterozygous prothrombin gene mutation |
RR 2–3 |
| Acquired – strong |
Fracture of hip or leg
Hip or knee replacement
Major general surgery with general anaesthesia ≥30 mins
Major trauma
Spinal cord injury |
Odds ratio >10 |
| Acquired – moderate |
Surgery with general anaesthesia <30 mins
Chemotherapy
Chronic medical illness, such as heart or respiratory failure, inflammatory bowel disease
Combined oral contraceptive pill
Pregnancy/postpartum
Previous venous thromboembolism
Malignancy
Recent hospitalisation with an acute illness
Antiphospholipid syndrome |
Odds ratio 2–9 |
| Acquired – weak |
Immobility due to sitting (prolonged car or air travel)
Increasing age
Laparoscopic surgery
Obesity
Varicose veins |
Odds ratio <2 |
| *Relative risk (RR) for first venous thrombosis |
Diagnostic strategy
Clinical prediction rules
The signs and symptoms of PE can be nonspecific, and only approximately 20% of patients with a clinically suspected VTE have it objectively confirmed.12 Therefore, it is important to establish the likelihood of PE using a clinical prediction rule to reduce unnecessary radiological tests. The simplified Geneva and Wells scores (Table 2) are the most commonly used scoring systems, but because neither can safely exclude VTE alone, they should be combined with further criteria, including the D-dimer, to assess whether an individual requires imaging.13–15
| Table 2. Simplified Geneva15 and Wells14 scores |
| Simplified Geneva score for pulmonary embolism |
Simplified Wells score for pulmonary embolism |
| Age >65 years |
1 |
Clinical signs and symptoms of deep vein thrombosis |
1 |
| Surgery or fracture in the previous four weeks |
1 |
Immobility/surgery in the previous four weeks |
1 |
| Previous venous thromboembolism |
1 |
Previous venous thromboembolism |
1 |
| Haemoptysis |
1 |
Haemoptysis |
1 |
| Active cancer |
1 |
Malignancy |
1 |
| Unilateral leg pain |
1 |
Alternative diagnosis less likely than pulmonary embolism |
1 |
Heart rate
75–94 beats/min
>95 beats/min |
1
2 |
Heart rate >100 beats/min |
1 |
| Pain on lower leg deep vein palpation and unilateral oedema |
1 |
|
|
Scoring
Low
Moderate
High
Unlikely
Likely |
0–1
2–4
≥5
0–2
≥3 |
Scoring
Unlikely
Likely |
0–1
≥2 |
Pulmonary embolism rule-out criteria rule
The PE rule-out criteria rule is a scoring system for excluding PE. It is applicable to patients aged <50 years and when the estimated rate of PE is low (<15%), such as in most Australian general practice and emergency departments.11,16 If one or more of the criteria are not met, PE cannot be ruled out and further assessment is required (Box 1 and Figure 1).
| Box 1. Pulmonary embolism rule‑out criteria16 |
- Age <50 years
- Pulse <100 beats per minute
- Oxygen saturation >95% on room air
- No exogenous oestrogen
- No prior venous thromboembolism
- No surgery or trauma in the previous four weeks
- No unilateral leg swelling
|
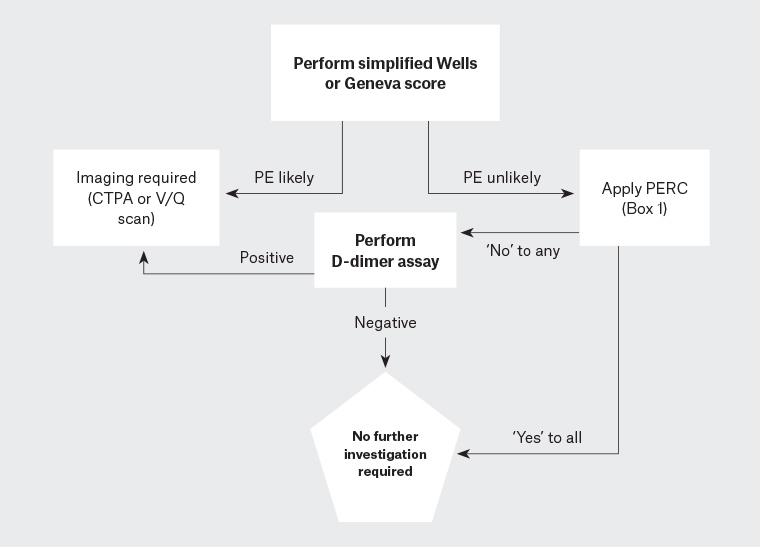
Figure 1. Suggested diagnostic algorithm for PE41
CTPA, computed tomography pulmonary angiography; PE, pulmonary embolism; PERC, pulmonary embolism rule-out criteria; V/Q scan, ventilation-perfusion scan; VT, venous thromboembolism
D-dimer assay
D-dimer is a degradation product of cross-linked fibrin, which has high sensitivity but low specificity for acute VTE – it is almost always elevated in acute thromboses but also in other conditions including sepsis, malignancy, pregnancy and trauma.17 A low Wells score and a negative D-dimer can safely and efficiently exclude PE in primary care without the need for imaging.18,19 Those with a high Wells or Geneva score should proceed directly to imaging.14,15
Imaging and further assessment
Figure 1 outlines a suggested diagnostic algorithm for PE. Computed tomography pulmonary angiography (CTPA) is preferred in most patients as a negative scan excludes PE, it has a low rate of inconclusive results and it may provide an alternative diagnosis if PE is excluded.20 A ventilation/perfusion (V/Q) scan is suitable for patients with renal impairment and is preferred for pregnant women; however, up to 55% of V/Q scans are non-diagnostic and require serial lower limb ultrasonography or CTPA to exclude PE.21 Investigation of suspected PE in pregnancy is a special scenario because of the overlap of symptoms of PE with physiological symptoms of pregnancy, an inability to use ‘standard’ pre-test probability scores, and considerations of radiation exposure to both the mother and fetus.22
Lower limb doppler ultrasonography has a high positive predictive value but low sensitivity and low negative predictive value for PE. It should only be used for non–high risk patients with relative or absolute contraindications for CTPA.23 Ultrasonography alone is not able to rule out PE. Echocardiography can be used to aid diagnosis in haemodynamically unstable patients with a high clinical probability of PE by detecting right ventricular dysfunction, particularly if the patient is too unstable to proceed to computed tomography.11
Considerations in primary care
Many of these algorithms were designed for use in emergency departments, with ready availability of laboratory testing and radiological imaging. In patients with a high pre-test probability, prompt referral for evaluation in the emergency department is warranted. If there are delays in definitive investigation and bleeding risk is low, an initial therapeutic dose of low molecular weight heparin (LMWH) should be considered.24
Management
Anticoagulation
Anticoagulation is indicated in almost all cases of PE because it is highly effective in preventing thrombus extension or recurrence.25 A trial in the 1960s showed that 50% of untreated (ie not anticoagulated) patients with PE either died or had recurrence, compared with 2% who were treated.26 One area of controversy is whether patients with isolated subsegmental PE require anticoagulation. People at high risk of recurrence, such as those with cancer, should be anticoagulated; individuals with a low risk of recurrence and no proximal DVT, or with a high bleeding risk, can be monitored without anticoagulation.25 It is recommended that a haematologist or respiratory physician be involved in the decision making.
Direct oral anticoagulants (DOACs) are now first-line treatment for most patients as they do not require routine monitoring, have few medication and food interactions and have a lower bleeding risk than warfarin.24 Warfarin may be preferred in those who are morbidly obese or have severe renal impairment, and it remains the anticoagulant of choice for antiphospholipid syndrome (APS).27,28 LMWH is indicated in pregnancy and breastfeeding as DOACs cannot be used safely.25 Most cases of cancer-associated thromboses can be treated with DOACs as large trials have shown similar efficacy and safety to LMWH; the exception is patients with unresected luminal genitourinary or gastrointestinal malignancy, in which case LMWH is still preferred because of a higher bleeding risk with DOACs.29,30
Duration of anticoagulation
All patients with PE should receive anticoagulant therapy for 3–6 months. In patients whose PE was provoked by a major reversible risk factor (such as those outlined in Table 1, ‘strong acquired risk factors’), anticoagulant therapy can be ceased at this point. Patients who have had two or more unprovoked VTEs or have a non-reversible risk factor, such as malignancy or APS, should continue anticoagulant therapy indefinitely. Those who have had a first unprovoked or minimally provoked PE are in a state of clinical equipoise for continuing anticoagulation beyond the initial 3–6 months, with the need to balance VTE recurrence risk with bleeding risk. VTE recurrence following unprovoked VTE following cessation of anticoagulation is approximately 10% at one year and 30% at 3–5 years, whereas bleeding risk in all-comers with DOACs is approximately 1% per year.24,31 It is important to gauge patient preference, but these authors tend to encourage patients with unprovoked VTE to continue anticoagulation indefinitely.
Role of thrombophilia testing and follow-up imaging
The presence or absence of an inherited thrombophilia does not modify initial management of acute PE. Furthermore, the influence of thrombophilia on recurrence risk, and therefore on decisions regarding duration of anticoagulation, is minimal in most situations.32 Therefore, thrombophilia testing is not recommended in most patients at presentation and may best be deferred to the haematologist. Those more likely to have a thrombophilia are younger (aged <50 years) and with a family history. It is reasonable to test for APS in those with unprovoked PE as this would alter initial management (warfarin over DOAC) and duration of anticoagulation (lifelong).
Follow-up imaging, in the absence of new symptoms, should not be used to determine the duration of anticoagulation, as significant numbers (up to 50%) have residual thrombosis following 3–6 months of anticoagulation.33 Imaging at the end of the initial 3–6-month treatment period is performed to establish a new baseline for the patient.
Monitoring for complications
CTEPH is an uncommon complication of acute PE that is associated with significant morbidity and mortality, affecting up to 4% of patients with PE.1,33–35 It occurs because of persistent obstruction of pulmonary arteries that has not resolved despite at least three months of therapeutic anticoagulation.36 Progressive or non-resolving dyspnoea on exertion is the predominant symptom. Investigation with echocardiography and V/Q scanning to detect perfusion defects should be performed, and if CTEPH is suspected, the patient should be referred to a specialist pulmonary hypertension centre. Treatment involves anticoagulation, consideration of pulmonary endarterectomy and targeted medical therapies for pulmonary arterial hypertension.36
Patients receiving long-term anticoagulation require at least yearly review to ensure the benefits of continued anticoagulation continue to outweigh risks. Those receiving DOACs require 6–12-monthly renal and hepatic function.
Conclusion
Despite advances in diagnosis and management, PE remains a significant contributor to morbidity and mortality from cardiovascular disease. For the GP, a high degree of diagnostic suspicion must be maintained, as presenting symptoms and signs can be nonspecific. Diagnosis involves a combination of pre-test probability, D-dimer and imaging. Anticoagulation is the mainstay of management, and GPs increasingly will be asked to monitor patients on extended-duration anticoagulation.
Key points
- PE remains a significant contributor to avoidable cardiovascular death.
- Diagnosis relies on an index of clinical suspicion, as symptoms and signs are nonspecific.
- A combination of clinical prediction tools and D-dimer can be used to decide which patients require further investigation with radiological imaging.
- The mainstay of management is anticoagulation, usually with DOACs, for at least three months.
- Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare but debilitating complication of acute PE.