Heart failure (HF) is a common yet complex chronic illness that affects approximately one in 200 adult Australians.1 The modern dichotomy of HF with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF) has been adopted by the National Heart Foundation of Australia and is the major guide to medical treatment selection (Table 1).2 Both HFrEF and HFpEF remain highly morbid conditions, with four-year mortality rates of 41% and 32%, respectively.2
| Table 1. Heart failure classification |
| |
Diagnostic criteria |
Causative conditions |
| Heart failure with reduced ejection fraction (HFrEF) |
|
- Ischaemic cardiomyopathy
- Idiopathic dilated cardiomyopathy
- Familial cardiomyopathy
- Medication-induced cardiomyopathy
|
| Heart failure with preserved ejection fraction (HFpEF) |
- LVEF >50%
- Signs and symptoms of heart failure (dyspnoea, peripheral or pulmonary oedema)
- Echocardiographic features of HFpEF:
- left atrial enlargement
- pulmonary hypertension
- impaired diastolic fulling
- average E/e’ >14
|
- Hypertensive cardiomyopathy
- Cardiac amyloidosis
- Hypertrophic cardiomyopathy
|
| E/e’, echocardiographic marker of left ventricular filling pressure; LVEF, left ventricular ejection fraction |
In terms of its pathophysiology, HF is characterised by a decline in the heart’s pumping ability that exceeds innate compensatory mechanisms (Figure 1).
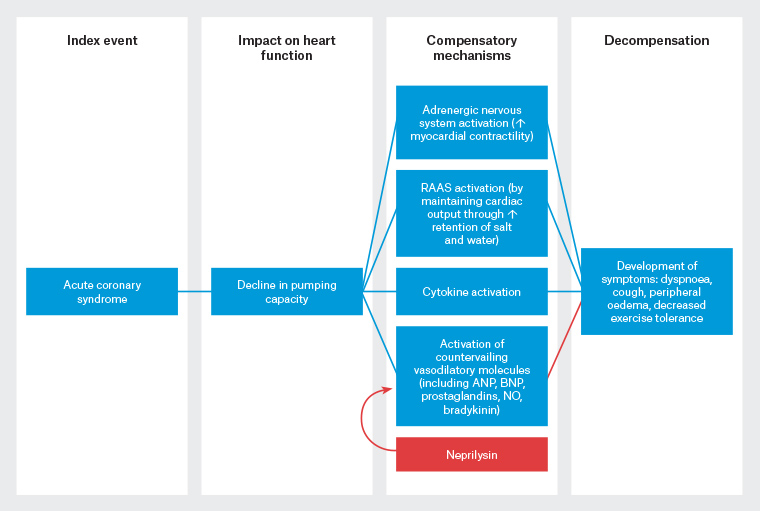
Figure 1. Heart failure (HF) begins after an index event (eg acute coronary syndrome) produces an initial decline in the heart’s pumping capacity. After this initial decline, various compensatory mechanisms are activated, including the activation of adrenergic signalling, upregulation of the RAAS and activation of vasodilatory cytokines. Importantly, many of these vasodilatory peptides, including bradykinin, ANP and BNP, are degraded by neprilysin, a membrane-bound peptidase. At some point, these compensatory mechanisms fail and patients decompensate, with an increase in overt symptoms of HF and a concurrent increase in mortality. Although the exact mechanisms that are responsible for this transition are unknown, the transition to symptomatic HF is accompanied by increasing activation of neurohormonal, adrenergic and cytokine systems that lead to maladaptive changes within the myocardium collectively, referred to as left ventricular remodelling.17 Click here to enlarge.
ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; NO, nitric oxide; RAAS, renin–angiotensin–aldosterone system
While options for treatment of HFpEF are limited, recent advances in medical therapy for HFrEF have changed the face of pharmacotherapy. Effective management of HF is best accomplished with a multidisciplinary team model including the cardiologist, specialist HF nurse, the general practitioner (GP), physiotherapist, dietitian and the patient/family/carers (Table 2).3
In this article, the authors provide an update on the role of GPs in coordinating and adjusting HF management, exploring the characteristics of the angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril/valsartan, and sodium–glucose co-transporter-2 (SGLT2) inhibitors. Both of these drug classes have been proven to reduce morbidity and mortality in landmark clinical trials.4–7
The aim of this article is to provide an update on the management of HF for GPs, who play a pivotal part in management of patients with complex presentations. It is hoped that this article will enable the increased uptake of ARNI and SGLT2 inhibitors, which will in turn improve patient quality of life and mortality outcomes.
| Table 2. A team approach to heart failure management |
| Patient |
- Adhering to medication
- Performing regular physical activity
- Monitoring weight and early symptom reporting
- Following a diuretic action plan to treat mild volume
overload/decompensation
|
| General practitioner |
- Coordinating care
- Providing patient and carer education that helps with improved understanding of heart failure and improved compliance with medications
- Up-titrating goal-directed heart failure medications
- Managing mild or early decompensation of heart failure (outpatient care)
- Identifying and treating reversible factors that exacerbate
heart failure
- Recognising and referring patients with deteriorating symptoms
- Supporting palliative care of patients with end-stage heart failure living in the community and within residential aged care facilities
|
| Heart failure nurse and cardiologist |
- Coordinating care
- Providing patient and family/carer education
- Up-titrating goal-directed heart failure medications
- Assessing and treating reversible causes of heart failure
- Instituting advanced therapies such as ivabradine, angiotensin receptor-neprilysin inhibitor, cardiac resynchronisation therapy, implantable cardioverter-defibrillator etc
- Determining end-of-life goals and facilitating engagement with palliative care or ‘supportive care’ clinics
|
| Physiotherapist |
- Assisting patients with cardiac rehabilitation
- Helping patients in maintaining and/or improving level of functioning with regular prescribed activities
|
| Dietitian |
- Helping patients in maintaining adequate nutritional status
- Encouraging dietary changes (eg low-salt diet, fluid restriction) to maintain euvolemia
|
Diagnosis and investigation of new onset or chronic heart failure
The first and critical step in HF management is to determine whether a patient who has been labelled as having HF can be classified as having either HFrEF or HFpEF. This is a direct determinant of treatment choices. HFrEF, formerly termed systolic HF, refers to patients with a left ventricular ejection fraction (LVEF) of less than 50%. HFrEF usually occurs in the setting of dilated cardiomyopathies and may be ischaemic or non-ischaemic in origin. HFpEF, previously known as diastolic HF, is diagnosed when there are clinical features of HF, a preserved LVEF of greater than 50% and echocardiographic features of HFpEF. These include left ventricular hypertrophy, left atrial dilatation, pulmonary hypertension and impaired diastolic filling. In patients with a previous ‘label’ of congestive cardiac failure or undifferentiated dyspnoea, re-stratification of their HF phenotype is crucial to guide further management. The New York Heart Association (NYHA) functional classification is a standardised tool for grading the severity of dyspnoea symptoms, and HF medication therapy should be maximised in patients with HFrEF whose dyspnoea is classified as NYHA II or above (Table 3).
| Table 3. New York Heart Association functional classification of heart failure |
| Class I |
No limitation of ordinary physical activity |
| Class II |
Slight limitation of ordinary physical activity. No symptoms at rest |
| Class III |
Marked limitation of ordinary physical activity. No symptoms at rest |
| Class IV |
Symptoms on any physical activity or at rest |
Standard pharmacological management of heart failure
The foundation of mortality reduction in HFrEF is treatment with a ‘triple therapy’ backbone including an HF-specific β-blocker, a renin–angiotensin system antagonist and a mineralocorticoid receptor antagonist (MRA). This triad of therapies is estimated to reduce three-year mortality by up to 56% in patients with HFrEF.8 Titrating up to target doses of the backbone therapy is essential and is best achieved in cooperation between the cardiologist and the GP. A timeline of clinical trials showing the efficacy of key mortality-reducing medications in the treatment of HFrEF is shown in Figure 2.
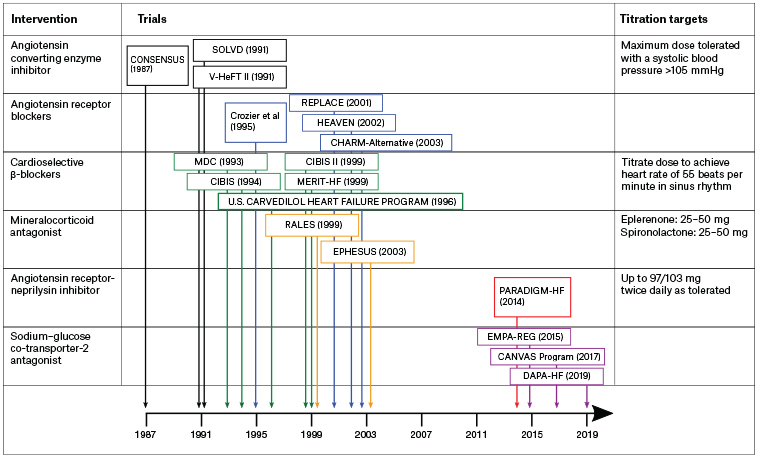
Figure 2. A timeline of clinical trials showing the efficacy of key mortality-reducing drugs in the treatment of heart failure with reduced ejection fraction.4–7,18–28 In these trials, angiotensin converting enzyme inhibitors were shown to produce a reduction in cardiovascular mortality of 14%, while β-blockers produced a mortality reduction of 32% and mineralocorticoid antagonists produced a mortality reduction of 28%. The titration targets for these interventions are shown alongside the drug classes. Click here to enlarge.
Angiotensin receptor-neprilysin inhibitor
Sacubitril/valsartan is the prototypical ARNI, which combines an angiotensin receptor antagonist with sacubitril. Sacubitril is an inhibitor of neprilysin, a membrane-bound peptidase that degrades the vasodilatory peptides bradykinin, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). By inhibiting the breakdown of ANP and BNP, sacubitril promotes vasodilation, natriuresis and aquaresis (ie excretion of water without electrolyte loss). The landmark randomised trial PARADIGM-HF showed a significant mortality benefit of an ARNI over enalapril in patients with HFrEF, with a number needed to treat (NNT) of 35 to prevent death and 21 to prevent the composite of cardiovascular death or first HF hospitalisation over a median of 27 months follow-up.4
Sacubitril/valsartan is currently listed on the Pharmaceutical Benefits Scheme (PBS) for patients with HFrEF (of less than 40%) and persisting HF symptoms (NYHA classes II, III or IV [Table 3]) despite maximum conventional therapy including a β-blocker and angiotensin converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB). Evidence-based guidelines suggest that sacubitril/valsartan should be reserved for patients with HF symptoms (NYHA classes II, III or IV) on maximal tolerated doses of triple therapy with a β-blocker, ACEI/ARB and MRA for at least 3–6 months.2
The PIONEER-HF randomised controlled trial of ARNI initiation in acute decompensated heart failure found ARNI initiation was well tolerated and was associated with a decrease in HF hospitalisation; however, the trial was not powered to detect a decrease in clinical endpoints.9 In these authors’ practice, earlier ARNI initiation may be considered in younger patients with severe left ventricular dysfunction or in patients with HFrEF and poorly controlled hypertension.9
Recommended prescribing practices for ARNI:2
- Usually started at low or moderate doses and uptitrated by doubling the dose every 2–4 weeks, aiming for target doses or maximum tolerated doses (Table 4). Uptitration of ARNIs should not affect commencing other medications (β-blockers and MRAs).
- As a result of the diuretic and hypotensive effect of sacubitril, it is also recommended to reduce loop diuretic dose by 25–50% when sacubitril/valsartan is introduced or uptitrated.10
- Sacubitril/valsartan must not be co-administered with any other ACEI or ARB.
- To avoid bradykinin-mediated side effects of cough and angioedema, a 36-hour washout period is required if changing from an ACEI to an ARNI. No washout period is required when changing from an ARB to an ARNI. If the patient develops angioedema, ARNI should be ceased and cardiologist advice sought.
- Patients should be reviewed following initiation and each dose escalation with monitoring of blood pressure and blood biochemistry (renal function and potassium) at 1–2 weeks and six-monthly in the long term.
- Caution should be exercised in patients with higher risk of symptomatic hypotension and falls, such as older, frail patients and those with pre-existing postural dizziness. In patients with baseline systolic blood pressure of <100 mmHg, the risk of symptomatic hypotension may outweigh benefits.
- Initiating therapy at a lower dose is recommended in patients over 75 years of age, patients at high risk of hypotension, patients with estimated glomerular filtration rate (eGFR) <60 mL/min, patients with moderate hepatic impairment (Child–Pugh class B) or if aspartate transaminase/alanine aminotransferase is more than twice the upper limit of normal.
| Table 4. Dose titration when commencing sacubitril/valsartan29 |
| Current ACEI/ARB dose |
Initial sacubitril/valsartan dose |
Sacubitril/valsartan titration |
| Full dose |
49/51 mg BD |
Increase dose after 2–4 weeks to target dose of 97/103 mg BD (or as tolerated) |
| Low dose |
24/26 mg BD |
Double dose every four weeks to target dose of 97/103 mg BD (or as tolerated) |
| ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BD, twice daily |
Minor side effects of ARNIs are similar to those of ACEIs/ARBs, including cough, hyperkalaemia and rise in serum creatinine. In randomised trials, the incidence of creatinine elevation and hyperkalaemia is significantly lower with ARNIs when compared with ACEIs or ARBs.4 Patients with eGFR of <30 mL/min/1.73m2 were not enrolled in landmark trials, and cardiologist guidance should be sought for these patients.
Sodium–glucose co-transporter-2 inhibitors
SGLT2 inhibitors are oral agents initially developed for the treatment of diabetes that have been shown to have dramatic benefits in patients with HFrEF. By inhibiting the sodium–glucose co-transporter in the proximal tubule, these agents inhibit reabsorption of both glucose and sodium, resulting in net excretion of sodium, glucose and water. The unexpected cardiovascular benefits were revealed in mandatory safety trials, which showed significantly reduced all-cause and cardiovascular mortality in patients with type 2 diabetes at high cardiovascular risk, with NNTs of 38 and 45, respectively, with empagliflozin.5 Subsequent dedicated randomised trials6 have shown that dapagliflozin may also reduce mortality and HF hospitalisations for patients with HFrEF, even in the absence of diabetes.
Dapagliflozin has recently been listed on the PBS for patients with HFrEF with NYHA class II, III or IV symptoms as an add-on therapy to optimal standard chronic HFrEF therapy. Dapagliflozin and empagliflozin are also PBS-subsidised as an additive agent for patients with type 2 diabetes and glycated haemoglobin (HbA1c) of >7.0% on either metformin, a sulphonylurea or insulin.
As a result of the significant reductions in HF hospitalisation and mortality, patients with diabetes and HFrEF meeting the aforementioned criteria should be strongly considered for introduction of SGLT2 inhibitors. In patients with good baseline glycaemic control (HbA1c <8.5%) or a history of hypoglycaemic events, guidelines recommend reducing insulin doses by 20% and ceasing sulphonylureas or glitazones when initiating SGLT2 therapy to reduce the incidence of euglycaemic diabetic ketoacidosis and hypoglycaemia.11,12 The usual dose of dapagliflozin is 10 mg once daily; for empagliflozin, it is 10 mg once daily as a starting dose, with a maximum dose of up to 25 mg daily.
As with ARNIs, SGLT2 inhibitors have a modest diuretic effect that is proportional to blood glucose levels. In patients who are euvolaemic and normotensive, loop diuretic doses should be reduced at the time of introduction by 25–50%, particularly in patients with poor baseline glycaemic control.12 Patients must be counselled to be vigilant for symptoms of genitourinary infections, which occur more frequently with SGLT2 inhibitor use. The most serious risk of SGLT2 inhibitors is euglycaemic ketoacidosis,13 which is most commonly precipitated by intercurrent illness or reduced oral intake. A ‘sick day plan’ should be discussed, and patients should be advised to withhold the medication, closely monitor blood glucose levels and ketones, and maintain good hydration while unwell.14
Managing heart failure in patients with chronic kidney disease
Balancing comorbid chronic kidney disease (CKD) with HF therapy is often difficult. In such patients, regular monitoring of serum creatinine, eGFR and potassium is necessary to prevent adverse effects of hyperkalaemia. In patients with stage 4 and 5 CKD, specialist nephrologist referral is often required, and MRAs should be avoided.15 Australian guidelines recommend tolerating a decrease in eGFR of up to 30% with the introduction or uptitration of renin–angiotensin system inhibitors;2 however, continued decline in renal function or serum potassium levels of >5.5 mmol/L should prompt dose reduction.2 A more cautious approach is warranted in elderly patients and those with advanced CKD.15
Frusemide remains an essential tool to maintain euvolaemia in patients with HF; however, use of prognosis-improving agents with diuretic effect – such as MRAs, sacubitril/valsartan and SGLT2 inhibitors – may facilitate reduction or even cessation of maintenance frusemide doses.16 Non-pharmacological measures remain crucial in fluid balance maintenance. Continuing education and emphasis must be made on the importance of modest fluid intake restriction (2–3 L per day) and regular monitoring of patient weight at home. An action plan should be developed to either take a short course of increased oral frusemide or seek early GP review in the event of increasing dyspnoea, weight or peripheral oedema.
Conclusion
Managing HF requires careful, continuous monitoring by various stakeholders: GPs, cardiologists, HF nurses and the patient. With new promising options including ARNIs and SGLT2 inhibitors available, we can hope to improve morbidity and mortality in patients with HF (mainly HFrEF), providing us with four pillars of HF therapy including ARNIs, β-blockers, MRAs and SGLT2 inhibitors.