Infertility affects 15% of people wishing to conceive, with profound consequences for the individuals, their families and the community if unresolved. In Australia, we are fortunate to be able to offer many patients ready access to comprehensive assessment and a range of highly successful treatments. Conception difficulties are a common reason for presentation to general practitioners (GPs), who play an integral part in both validating patients’ concerns and managing their care. This includes advice for optimisation of trying to conceive, lifestyle modification, timely and relevant investigations and referral to non-GP specialist care. Optimisation of reproductive health and advances in assisted reproductive technology (ART) have both contributed to improvements in the safety and efficiency of treatment, aiming for healthy singleton live birth.1
Defining the problem and its prevalence: Is infertility actually rising?
Infertility is a public health problem affecting over 180 million people globally.2,3 One in six couples experiences infertility, requiring fertility advice; however, this rises to 1:3 couples aged in the late 30s and 1:2 aged in the early 40s.4 Traditionally, infertility has been defined as the failure to achieve pregnancy within one year of regular, unprotected intercourse.5 However, this definition lacks nuance, overlooking the importance of a woman’s age, regular ovulation and timing of intercourse to optimise conception chances. Some people have infertility because they need a sperm donor, egg donor and/or a surrogate to conceive.
The prevalence of infertility appears to be increasing. As well as the obvious contributions from increasing maternal age with delayed reproduction, environmental risks and general health impacts,6,7 it is likely there is less reticence for seeking help and greater awareness of the availability of effective assistance.8
Age
Female age is the single most important factor affecting the chance of successful conception (Figure 1), with deleterious effects on chromosomes, genes, DNA repair and mitochondrial function with increasing age.9 This is particularly impactful given the major societal trend towards delayed childbearing.10 One-quarter of Australian women giving birth are aged ≥35 years, with 29% of those being first-time mothers. Since 1999 the rate of women aged 40–44 years giving birth has almost doubled.11
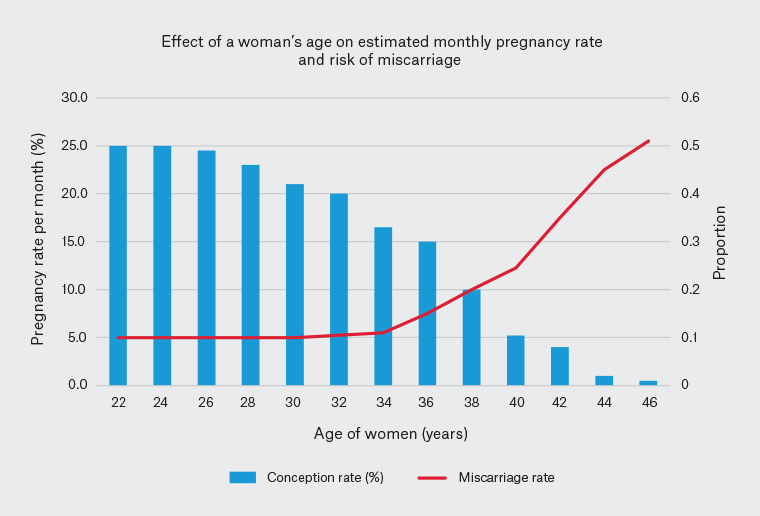
Figure 1. Fertility and miscarriage rates as a function of maternal age32,33
This is predominantly due to lack of opportunity for having a family with a partner rather than intentional delay.12
Not just a female problem
In men the fertility decline starts later and is more subtle. Quality and quantity of sperm become noticeably reduced after the age of 45 years.13,14 The clinical impact is still not well quantified; however, the risk of chromosomal abnormalities, congenital abnormalities and neuropsychological conditions such as autism spectrum disorder, epilepsy and schizophrenia all increase in the offspring of men who conceive a child after the age of 45 years.13
Emerging evidence demonstrates that infertile men are predisposed to a greater burden of associated disease (including heart disease).15 Potential causes of the observation that semen parameters are declining over time include exposure to environmental endocrine disrupting chemicals (eg plasticisers, bisphenol A and phthalates), rising rates of obesity and the trend of delayed parenthood.6,13,16
The workup: How good are our tests?
While the causes of infertility are many, varied and often multifactorial, the tests routinely employed in clinical practice are relatively unsophisticated, with little improvement over the past 10–20 years (Table 1).
| Table 1. Commonly used tests to assess fertility |
| Ovulation |
- Urine testing for luteinising hormone surge, which occurs the day before ovulation
- Ultrasonographical surveillance of growing leading follicle
- Serum progesterone testing, which starts to rise at ovulation and is maximal seven days after ovulation (persists in pregnancy but starts to drop after the midluteal phase if not pregnant)
|
| Other hormone assessments as required |
- Testosterone
- Sex hormone binding globulin
- Anti-Müllerian hormone
- Thyroid-stimulating hormone
|
| Pelvic assessment |
- Pelvic ultrasonography to visualise ovaries and uterus
- Detailed or specialised ultrasonography can suggest deep infiltrating endometriosis
- Pelvic ultrasonography with fluid introduced into uterine cavity and fallopian tubes to demonstrate tubal patency (sonohysterography and tubal patency test)
- Hysteroscopy and laparoscopy
|
| Sperm testing34 |
- Volume (>1.5 mL)
- Concentration (>15 M/mL)
- Progressive motility (>32%)
- Morphology (>4%)
- Antisperm antibodies
|
In women with regular cycles, ovulation occurs in at least 90% of cycles, and ovulation monitoring is often unnecessary. Home testing, such as urine luteinising hormone (LH), body temperature and mucus, may not always be reliable. Some women find home tests helpful, while for others they provoke undue stress and anxiety and should be avoided. For women with menstrual irregularity, hormonal assessment – such as day 2–3 follicle-stimulating hormone (FSH), LH, oestradiol, thyroid function, prolactin and androgens if polycystic ovarian syndrome (PCOS) is suspected – is recommended.17,18 Awaiting day 2–3 for those with very infrequent periods is impractical, and these blood tests can be done at any time.
Improvements in ultrasound technology in specialised centres facilitate non-invasive diagnosis of deep infiltrating endometriosis in addition to other pelvic pathology and antral follicle count. An sonohysterography improves visualisation of the endometrium and uterine pathology as well as being used for diagnosis of tubal patency. A hysterosalpingography X-ray can be requested if there is limited access to ultrasonography. A laparoscopy for diagnosis and treatment of endometriosis can be beneficial (and outweigh risks of laparoscopic surgery) in the presence of significant pain symptoms or other pelvic pathology, or after detailed discussion of available fertility treatments.
A semen analysis, which is performed in a specialised andrology laboratory, can also provide information about antisperm antibodies, which can reduce chances of natural conception.19 The concentration and motility of sperm are the most important parameters for spontaneous conception, and morphology is least predictive. There is variability in semen parameters, and men with a result outside of the reference range should be retested after at least 2–3 months because of the progression of spermatogenesis.
Timely referral is the key to best outcomes, and for young patients without obvious risk factors, it may be appropriate to refer after 12 months of trying to conceive. For women aged over 35 years and those with other risk factors for infertility – including irregular ovulation, pelvic pathology, likely male infertility or a family history of premature ovarian insufficiency – referral after six months, or even immediately, may be appropriate.
Anti-Müllerian hormone: What is it actually telling us?
Anti-Müllerian hormone (AMH) has been unfortunately and somewhat incorrectly viewed as a test that can reveal a woman’s fertility potential, which has led to many of these tests being ordered following patient request. Accumulated evidence convincingly shows that the AMH result cannot predict a woman’s fertility, window of time to pregnancy or chance of a successful pregnancy.20 This makes the interpretation, without provoking anxiety or alternatively providing false reassurance, sometimes difficult, even for fertility specialists.
AMH is secreted by the granulosa cells of follicles in a woman’s ovaries, which will be recruited in the subsequent few menstrual cycles and have been shown to correlate well with the number of primordial (or resting) follicles. There is enormous variability in the results (Figure 2) for women in the reproductive age group.21 The levels diminish with age, and AMH is a marker of long-term ovarian function. In contrast, early follicular phase FSH assesses more immediate function and fluctuates throughout the menstrual cycle. While AMH levels can be measured during hormone use, long-term combined oral contraceptive pill use can suppress AMH.
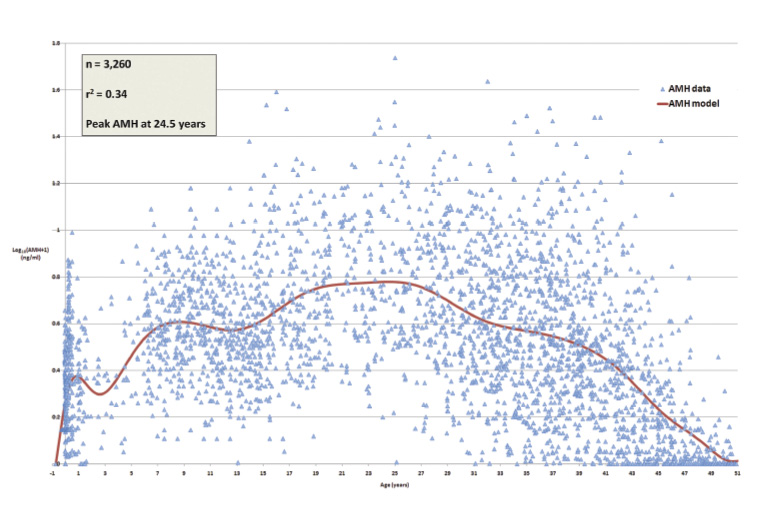
Figure 2. Anti-Müllerian hormone (AMH) variability from conception to menopause. Click here to enlarge
Reproduced from Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH, A validated model of serum anti-müllerian hormone from conception to menopause, PLoS One, 2011;6(7):e22024. doi: 10.1371/journal.pone.0022024. Licenced under the the Creative Commons Attribution License.
The main utility of AMH assessment is in infertility practice to predict ovarian response to ovarian stimulation and guide gonadotropin dosing. It can also be used to assess ovarian reserve following cancer treatment.22 Outside of these areas, it is rarely helpful and often unnecessarily causes distress.21
Fertility treatment options
For patients with good prognosis – such as those at a younger age and with a short duration of trying to conceive and good ovarian reserve – minimally assisted measures such as lifestyle intervention and optimisation of coital timing via cycle tracking may be useful starting points. However, for those with specific infertility factors, advanced age or protracted infertility, more efficient strategies such as in vitro fertilisation (IVF)/intracytoplasmic sperm injection are recommended at the start to avoid delay. Table 2 describes the common methods used for fertility assistance.
| Table 2. Fertility assistance methods |
| |
Ovulation induction |
Ovulation induction with intrauterine insemination |
In vitro fertilisation |
| Indications |
- Irregular ovulation
- Anovulation
|
- Mild infertility with normal sperm and patent tubes
- Difficulty with sexual intercourse
- Requirement for donor sperm
|
- Pelvic pathology, such as endometriosis and tubal factors
- Poor sperm
- Significant infertility (age, duration)
|
| Methods |
- Selective oestrogen receptor modulator and aromatase inhibitors, such as clomiphene and letrozole
- Follicle-stimulating hormone (and sometimes luteinising hormone) subcutaneous injections
|
- Tablets or injections as for ovulation induction
- Sperm preparation and insertion through the cervix
|
- Ovarian stimulation with injections
- Transvaginal ultrasound-guided oocyte retrieval (under sedation or general anaesthetic)
- Fertilisation of oocytes in the lab
- Embryo transfer 2–5 days later
|
| Success |
- 40–80% ovulation rates
- 15–20% pregnancy rates per cycle
|
- 5–15% pregnancy rate per cycle
|
- Pregnancy rates depend on maternal age
- 35–45% per embryo transferred (clinical pregnancy rate for women aged <39 years)
|
| Risks |
- Multiple pregnancies
- Hyperstimulation
- An authorisation code is required for prescription of clomiphene, to ensure monitoring of cycle, which has multiple benefits including multiple pregnancy risk reduction
|
- Multiple pregnancies
- Hyperstimulation
|
- Ovarian hyperstimulation syndrome
- Thrombosis
- Ovum pick up risk, bleeding and infection
- Multiple pregnancies if more than one embryo transferred
|
IVF: Standards of success
IVF treatment is strongly regulated in Australia.1 There are many ways to express pregnancy rates in ART, using various descriptors such as implantation, clinical pregnancy and live birth rates. Comparison of results from different clinics is extremely difficult and has led to the government-funded, direct-to-consumer Your IVF Success website
(www.yourivfsuccess.com.au). This website shows clinic success rates based on the data provided by clinics to ANZARD (Australia and New Zealand Assisted Reproduction Database). However, it is important to be aware of limitations to the information provided. There are nuances regarding the types of patient population that a clinic treats, which can significantly affect success rates.
Implantation rates (the chance of a clinical pregnancy per embryo transferred) are approximately 35–45% for women aged <38 years, and it often takes multiple attempts to achieve a pregnancy. From the latest Victorian statistics, the cumulative live birth rate per woman after up to three stimulated cycles was 65% for women aged up to 30 years and dropped to 11% for women aged 42–43 years (VARTA report 2020/2021).23
Enhancing treatment safety
Single embryo transfer
A continuing trend in ART treatment in Australia has been the reduction in the rate of multiple births, from 4.9% in 2014 to 3.2% in 2018.24 This has been achieved as a result of a coordinated effort by GPs, clinicians, nurses and counsellors educating patients on the benefits of single embryo transfer, with the proportion increasing from 79.2% in 2014 to 90.6% in 2018 (ANZARD report 2019, https://npesu.unsw.edu.au/surveillance/assisted-reproductive-technology-australia-and-new-zealand-2019).25
Ovarian hyperstimulation syndrome reduction
For at-risk patients, a ‘freeze-all’ cycle (with no fresh embryo transfer) using a gonadotropin-releasing hormone agonist trigger substantially reduces risk of ovarian hyperstimulation syndrome without significantly compromising the ultimate chance of success.26
Lab: State of the ART
Apart from the obvious goal of increasing live birth rates, improving outcomes in ART more broadly focuses on helping more patients become parents of healthy children in the shortest time possible. To achieve this, emphasis is placed on purpose-built laboratories, with the health of gametes and embryos in mind.27
Expanding horizons
Beyond infertility, ART can be used successfully in the following settings.
Pre-implantation genetic testing for monogenic disorders and structural rearrangements
Genetic carrier screening identifies couples at risk of serious genetic diseases, such as cystic fibrosis, so they may choose to undergo embryo biopsy and testing, with the goal of reducing the risk of having an affected child.
Fertility preservation
There are fertility-preserving options available for females and males affected by cancer or serious medical conditions to provide future options for having genetically related offspring. These options include egg and embryo freezing, ovarian tissue freezing (which can also be performed for prepubertal females) and medical ovarian suppression.28 For men, mature sperm freezing is readily available, while testicular tissue freezing (which is still experimental) is available for prepubertal boys.29
Elective oocyte freezing
As a result of the profound impact of age on oocyte quality (and ovarian reserve), elective egg freezing is increasingly used by single women for additional opportunities to conceive in the future if they are unable to do so spontaneously.30 While studies report that only a small proportion of women have returned to use their frozen eggs,31 there may be temporal delay, with some women still to use these eggs in the future to complete (if not start) their families. For those not requiring their eggs, there is an option to donate them to other women or couples who require donor eggs for conception.
Same sex couples and single women
There is a high demand for donor sperm to assist single women (53%), women in same-sex relationships (34%) and heterosexual couples (17%) to start their families (VARTA report 2020/2021).23 Men who donate sperm are screened for infectious diseases, complete medical and genetic health questionnaires and undergo genetic carrier screening. This offers useful information to the patient and offspring. The donor’s identifying information is stored in VARTA’s Central Register so the child can access information and potentially contact their donor in the future (VARTA donor register, www.varta.org.au/donor-conception-register-services).
Conclusion
Many options for fertility assistance are available for patients and couples who face fertility struggles. GPs can assist patients with general reproductive health and lifestyle optimisation, timely and relevant investigations, as well as prompt referral. Good communication between the fertility specialist and GP is important to nurture, as it enhances patient satisfaction.