Chronic kidney disease mineral and bone disorder (CKD-MBD) is an important complication of chronic kidney disease (CKD). Patients with CKD-MBD have an increased incidence of fracture, cardiovascular complications and mortality.1,2 The Kidney Disease: Improving Global Outcomes (KDIGO) group released a clinical practice guideline update in 2017 based on novel evidence in preceding years.3 The aim of this article is to provide an update and overview of CKD-MBD.
Definition and pathogenesis
KDIGO defines CKD-MBD as ‘a systemic disorder of mineral and bone metabolism due to CKD manifested by either one or a combination of the following: abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism; abnormalities in bone turnover, mineralization, volume, linear growth, or strength; or vascular or other soft tissue calcification’.4 Patients are at greater risk of mineral and bone metabolism disturbances from stage 3a CKD onwards (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73m2).
Pathogenesis is complex, involving feedback mechanisms between phosphate, calcium, PTH, vitamin D and recently described key players (Figure 1).
5–7 Renal dysfunction leads to phosphate retention. Retained phosphate leads to downregulation of calcitriol production, which leads to hypocalcaemia. Reduced renal production of 1-alpha-hydroxylase (which is required to produce calcitriol, or activated vitamin D) also contributes to decreased calcitriol. Changes are exacerbated by under-expression of parathyroid calcium-sensing and vitamin D receptors.
7 These changes stimulate PTH secretion, leading to secondary hyperparathyroidism. In recent years, the identification of fibroblast growth factor 23 (FGF-23) has advanced the understanding of CKD-MBD.
8 FGF-23 levels rise as CKD progresses. FGF-23, derived from osteocytes, requires Klotho (a transmembrane protein) to bind its target receptor. Elevated levels are independently associated with cardiovascular risk and mortality.
9 FGF-23 enhances phosphate excretion and downregulates calcitriol production. Resulting hypocalcaemia further enhances PTH secretion. Excessive PTH leads to release of calcium from bone. Emerging research also suggests a role for inhibition of the Wnt pathway in the pathogenesis of CKD-MBD, particularly by Dickkopf-related protein 1 and sclerostin.
10 Although detailed discussion is outside the scope of this article, these may present future therapeutic targets.
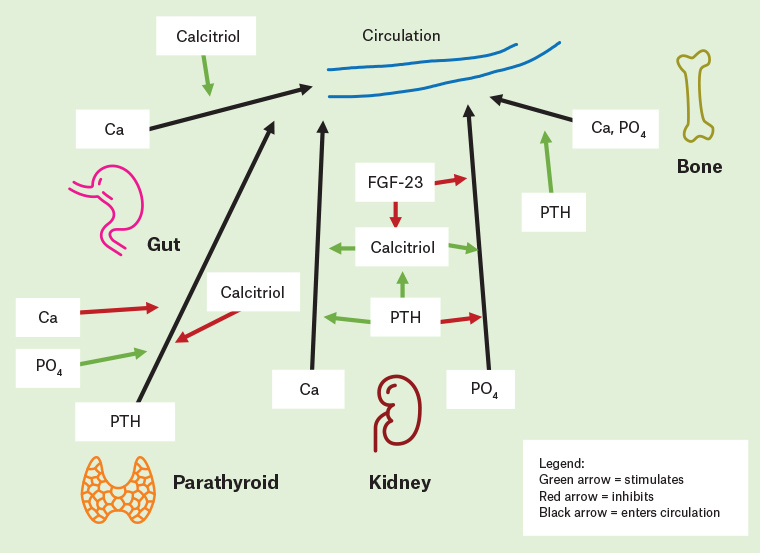
Figure 1. Simplified diagrammatic representation of chronic kidney disease mineral and bone disorder pathogenesis and the roles of calcium, phosphate, parathyroid hormone, fibroblast growth factor-23 and calcitriol. It should be noted that calcitriol can be endogenous or exogenous (eg in the form of calcitriol used to treat secondary hyperparathyroidism).
Ca, calcium; FGF-23, fibroblast growth factor-23; PTH, parathyroid hormone; PO4 , phosphate
Management
Managing CKD-MBD relies on controlling the balance between calcium, phosphate and PTH. KDIGO guidelines recommend treatment based on serial measurement of these markers considered together.3 Treatment and monitoring varies on the basis of CKD stage. Management of patients undergoing dialysis and those who have had a transplant is a specialised area that may differ from the pre–renal replacement therapy population.
Monitoring
Monitoring of serum biochemical markers should begin in CKD stage 3a. Frequency depends on presence and severity of abnormalities, CKD stage and rate of progression (Table 1). Monitoring can be increased if treatments are being given or abnormalities identified. Vitamin D (25-hydroxyvitamin D) may be measured and deficiency treated with cholecalciferol as for the general population.3 For renal transplant recipients, in the immediate post-transplant period, calcium and phosphate should be measured at least weekly until stable; after this, standard recommendations apply.3 Identified biochemical abnormalities in transplant recipients may be managed as per standard recommendations for CKD stages 3a–5.3
| Table 1. Kidney Disease: Improving Global Outcomes recommendations for chronic kidney disease mineral and bone disorder biochemical monitoring3 |
| Biochemical marker |
Frequency
stage 3a–3b
(eGFR 30–60 mL/min/1.73m2) |
Frequency
stage 4
(eGFR 15–30 mL/min/1.73m2) |
Frequency
stage 5 including dialysis/5D
(eGFR <15 mL/min/1.73m2) |
| Serum calcium and phosphate |
6–12 months |
3–6 months |
1–3 months |
| Serum PTH |
Based on baseline level and CKD progression |
6–12 months |
3–6 months |
| Serum ALP |
Not recommended |
12 months, or more often if elevated PTH |
12 months, or more often if elevated PTH |
| ALP, alkaline phosphatase; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone |
Phosphate levels
Hyperphosphataemia is associated with mortality in patients with CKD.11 However, there is no evidence that normalising phosphate improves clinical outcomes, and a previous trial revealed that the use of phosphate binders (both calcium and non-calcium based) led to a reduction of serum phosphate but increased coronary calcification score.12 KDIGO recommends lowering elevated phosphate levels toward the normal range for CKD stages 3a–5D, compared with the previous guideline of keeping within normal range for CKD stages 3a–5 and lowering towards normal for CKD stage 5D.3,13
Hyperphosphataemia is controlled with dietary restriction and phosphate binders. Daily phosphorus should be limited to less than 800–1000 mg/day14 by reducing intake of high-phosphate foods such as soft drinks and processed foods with phosphate additives. Food sources should be considered; efforts to reduce phosphate intake should not compromise nutrition. Intestinal absorptive capacity varies on the basis of the source of phosphate and is lower with plant-based phosphorus (<40%).15 Boiling may also reduce phosphorus content by demineralising food. Dietary counselling is highly effective, especially with a multidisciplinary approach, and the ‘phosphorus pyramid’ is a helpful tool.15
There are calcium-based and non–calcium based phosphate binders.16 Table 2 provides a summary of available phosphate binders. Calcium-based binders are listed on the Pharmaceutical Benefits Scheme (PBS) for hyperphosphataemia at any CKD stage and are inexpensive. They are generally used for patients who are not undergoing dialysis but may also be used for patients undergoing dialysis, especially if they are hypocalcaemic. The Kidney Disease Outcomes Quality Initiative guidelines have suggested the dose not exceed 1500 mg/day of elemental calcium; however, this recommendation has not been tested in clinical settings.14 Evidence suggests increased vascular calcification, cardiovascular risk and mortality when compared with non–calcium based binders.17 Aluminium-based binders are effective and inexpensive but not recommended for long-term use because of concerns about aluminium intoxication.3 Sevelamer, sucroferric oxyhydroxide and lanthanum are non–calcium based binders that are PBS-listed for patients undergoing dialysis. Binders should be given with meals as they limit gastrointestinal phosphate absorption. Adherence should be monitored as pill burden and gastrointestinal side effects render it challenging.18 Individualised pharmacist-led education has been shown to improve patient understanding and reduce concern regarding side effects.19 Conversion to an alternative binder or to chewable forms – such as chewable calcium carbonate, lanthanum or sucroferric oxyhydroxide – may be favourable for some patients. Sucroferric oxyhydroxide also has a lower pill burden than other binders, which may improve adherence.20
| Table 2. Summary of available phosphate binders16 |
| Binder |
Indication |
Starting dose |
Adverse effects* |
Other considerations |
| Calcium carbonate |
Hyperphosphataemia at all stages of CKD |
500–600 mg three times per day with meals |
Hypercalcaemia, vascular calcification |
Monitor serum calcium. |
| Aluminium hydroxide |
Hyperphosphataemia at all stages of CKD |
600 mg three times per day with meals |
Nervous system toxicity, gastrointestinal side effects |
May be used short term. Monitor serum aluminium concentration. Contraindicated in pregnancy. |
| Sevelamer |
Hyperphosphataemia in patients undergoing dialysis |
800–1600 mg three times per day with meals |
Gastrointestinal side effects, cough, dyspnoea |
Avoid if history of bowel obstruction; use with caution if pre-existing gastrointestinal motility disorder or major surgery. |
| Sucroferric oxyhydroxide |
Hyperphosphataemia in patients undergoing dialysis |
500 mg three times per day with meals |
Gastrointestinal side effects, tooth or tongue discolouration |
May cause black discolouration of faeces. Use with caution if iron accumulation disorder. Avoid if fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency. |
| Lanthanum |
Hyperphosphataemia in patients undergoing dialysis |
250–750 mg three times per day with meals |
Gastrointestinal side effects, hypocalcaemia |
Use with caution if pre-existing gastrointestinal disorder such as diverticular disease or motility disorder. Radio-opaque. |
*Common gastrointestinal side effects to these agents include nausea, vomiting, diarrhoea, constipation, abdominal pain.
CKD, chronic kidney disease |
Parathyroid hormone levels
Hyperparathyroidism is linked with mortality in patients with CKD.21,22 Elevated PTH may represent a compensatory response to increasing bone resistance to PTH, and treatment should be based on persistently high, progressively rising values rather than a single reading – a change from previous guidelines of treating isolated PTH levels above the normal limit.3 At all CKD stages, triggers for release should be controlled, including hyperphosphataemia, high phosphate intake, hypocalcaemia and vitamin D deficiency. Treating vitamin D deficiency with cholecalciferol has been shown to be effective and safe and lead to a reduction in PTH.23
Aggressive suppression of PTH increases the risk of adynamic bone disease, a low-turnover form of renal osteodystrophy.24 Recommendations for patients undergoing dialysis are to maintain PTH levels to within two-to-nine-fold the upper limit of normal.3 The optimal PTH level for patients not undergoing dialysis is unknown.3
Calcitriol and calcimimetics are used to treat hyperparathyroidism, primarily in patients undergoing dialysis, and may be used in combination.3 Calcitriol is a vitamin D receptor activator that suppresses PTH release (Figure 1). It can lead to hypercalcaemia and hyperphosphataemia. Calcitriol is recommended in severe, progressive hyperparathyroidism, started at low doses and titrated on the basis of PTH response.3 It is no longer routinely recommended in patients who are not undergoing dialysis in view of studies showing hypercalcaemia without improvement in clinically relevant outcomes.25 Calcitriol, which is PBS-listed for hypocalcaemia related to renal disease, is more commonly used in clinical practice to suppress elevated PTH.
Cinacalcet, a calcimimetic, reduces PTH by increasing sensitivity of the calcium-sensing receptor to extracellular calcium. It is PBS-approved for patients undergoing dialysis treated by a nephrologist. A common side effect is hypocalcaemia, which may be managed with dose adjustment or combination with low-dose calcitriol.7
In CKD stages 3a–5D, parathyroidectomy may be considered for secondary hyperparathyroidism refractory to medical management.3 Tertiary hyperparathyroidism occurs after longstanding secondary hyperparathyroidism and is related to parathyroid hyperplasia and autonomous PTH secretion. The definitive treatment is also parathyroidectomy.26
It is important to exercise caution with calcium-based phosphate binders and calcitriol. Both may cause hypercalcaemia, which should be avoided because of its link to mortality and cardiovascular events.27
Bone mineral density and use of antiresorptive therapy
CKD-MBD and osteoporosis may co-exist. KDIGO guidelines recommend bone mineral density (BMD) testing be considered in patients with CKD-MBD and/or risk factors for osteoporosis if it will affect treatment decisions.3 Bone biopsy is the gold standard for diagnosis of renal osteodystrophy and may be considered if knowledge of the type of osteodystrophy will affect treatment decisions.3,28 However, practically, this is invasive, difficult to obtain and not routine practice apart from exceptional circumstances with specialist nephrology and/or endocrine input. It is not a prerequisite for antiresorptive therapy.3 Serum PTH and bone-specific alkaline phosphatase can be tested to evaluate for bone disease; markedly high or low values reflect underlying bone turnover.29
Osteoporosis treatment varies on the basis of CKD stage. Patients with CKD stages 1–3 with normal PTH levels are managed as for the general population.3 Management is complex in advanced CKD (stages 4–5), where non-GP specialist input is important to develop an individualised approach. Transplant recipients have additional risk factors, including glucocorticoid exposure. Transplant recipients with eGFR >30 mL/min/1.73m2 with low BMD in the first 12 months may be treated with vitamin D and/or antiresorptives.3
Osteoporosis medications have not been studied in patients with CKD-MBD. Further research is needed regarding their role.30 Evidence suggests that current treatment paradigms for CKD-MBD have not significantly improved fracture rates.31
Denosumab is not renally cleared and may be used in CKD without dose adjustment.32 However, use in advanced CKD is complex because of increased risk of hypocalcaemia.33 Osteoporosis treatment with denosumab in various stages of CKD, including patients undergoing dialysis, has been shown to reduce fractures, and resultant hypocalcaemia is manageable with adequate calcium and vitamin D supplementation.34,35 Hence, denosumab may be considered in patients with advanced CKD and osteoporosis provided patients are aware of the risk of hypocalcaemia, and frequent monitoring and aggressive supplementation are undertaken.33 Patients should be calcium and vitamin D replete prior to initiation of treatment, and supplementation is required during treatment. Monitoring of calcium levels should continue 25–30 days post treatment, the approximate half-life of denosumab.32 In patients undergoing haemodialysis, another strategy used by nephrologists may be to increase dialysate calcium concentration.35
Bisphosphonates are poorly studied in CKD stages 4–5; they are renally cleared and routinely not recommended in patients with eGFR <30 mL/min/1.73m2.36 They may cause renal adverse effects, which are uncommon when lower (osteoporotic range) doses are used.37 Bisphosphonates may exacerbate low bone turnover states and should be avoided in these settings.38 Nevertheless, evidence suggests oral bisphosphonates are effective in increasing BMD and may be safely used in CKD stage 4.39,40 Data are further limited on bisphosphonates in patients undergoing dialysis, although a previous trial showed short-term, low-dose alendronate in patients undergoing haemodialysis to be well tolerated and preserve BMD.41 Data on dosing and schedule are lacking, but typically doses and administration frequency may be reduced from standard.37
In summary, these agents may be used in CKD stages 1–3 in patients with osteoporosis. For CKD stages 4–5, specialty input is warranted; denosumab may be considered if serum calcium can be monitored and controlled, and bisphosphonates may have a role on a case-by-case basis.
Conclusion
CKD is highly prevalent, and CKD-MBD is an important complication leading to poor outcomes.
Key points
- CKD-MBD is an important cause of morbidity and mortality, and monitoring for CKD-MBD markers should begin at stage 3a.
- Biochemical markers should be monitored serially, and management decisions based on trends rather than single readings, with phosphate lowered towards normal in CKD stages 3a–5D.
- Raised PTH may represent a compensatory mechanism, and in patients undergoing dialysis the target is two-to-nine-fold the upper limit of normal; optimal levels are unknown for patients not undergoing dialysis.
- Calcium-based phosphate binders and calcitriol should be used carefully given risks of harm.
- Managing osteoporosis in advanced CKD is complex, and antiresorptive therapy may be considered in some settings.