Thyroid cancer is becoming more common in Australia because of increasing incidence and decreasing mortality over the past 30 years.1 There has been a fourfold increase in the annual incidence of thyroid cancer in Australia since 1982, with current estimates suggesting 1.2% of Australians will be diagnosed with thyroid cancer by age 85 years. Differentiated thyroid cancers (DTCs) make up the majority of diagnoses and have an excellent overall survivorship, with a >97% five-year survival rate.1
Differentiated thyroid cancers arise from follicular cells, with papillary thyroid carcinomas (PTCs) and follicular thyroid carcinomas (FTCs) by far the most common types (Figure 1). Medullary thyroid cancers (MTCs) arising from the parafollicular C-cells are neuroendocrine tumours that are associated with familial and multiple endocrine neoplasia (MEN) syndromes. MTCs are distinct in their association with calcitonin and carcinoembryogenic antigen (CEA) tumour markers and have a different management guideline.2 A small proportion of cancers are undifferentiated anaplastic carcinomas that convey a very poor prognosis.
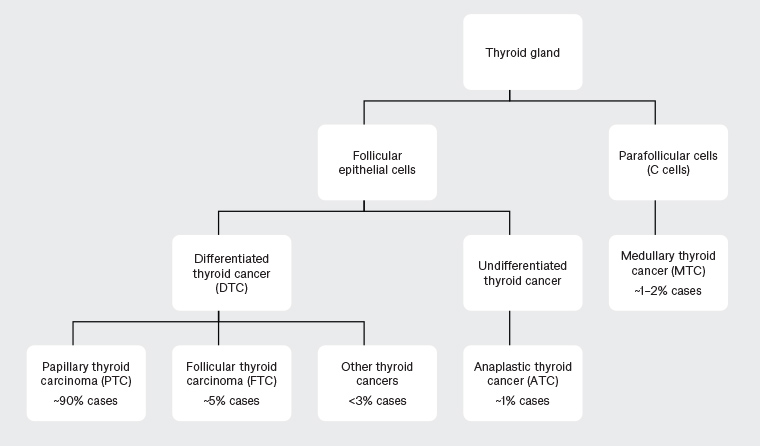
Figure 1. Types of thyroid cancer and proportion of cases14
Survivorship care is an integrated, patient-centred model that involves health professionals, the patient and their personal support network. The goals of survivorship care for DTC are the early detection of cancer recurrence, the maintenance of thyroid function with appropriate thyroid-stimulating hormone (TSH) suppression and the provision of symptom management and supportive care. In addition, preventive care and promotion of a healthy lifestyle are important factors in the survivorship of all cancer patients.3
Initial follow-up after primary treatment for DTC is usually with a specialist thyroid surgeon and/or endocrinologist governed by published guidelines.4,5 Thereafter, appropriate patients with DTCs may be returned to their general practitioner (GP) for shared care or follow-up in primary practice alone. A Canadian study found patients with low-risk DTC had similar outcomes when followed up in primary care when compared with thyroid specialist care and additionally had an economic benefit to the healthcare system.6
Risk stratification
The American Thyroid Association (ATA) divides DTC into low, intermediate and high risk of recurrence based on histological subtype, size and extent of the primary tumour and presence of distant metastases (Table 1).4 Within PTC, there are numerous histological variants including aggressive subtypes: tall cell, columnar, hobnail and solid. Conversely, non-invasive follicular thyroid neoplasm with papillary like features (NIFTP) is an indolent variant of PTC with <1% risk of recurrence.5
| Table 1. Classification of risk of thyroid cancer recurrence, and recommended initial TSH targets4 |
| Risk of recurrence |
Definition |
Initial TSH target* |
Low
(<5%) |
PTC with all of the following:
- Intrathyroidal tumour, size <4 cm, absence of gross extrathyroidal extension/vascular invasion/aggressive histology subtypes
- Complete macroscopic tumour resection
- No macroscopic locoregional or distant metastases
|
Hemithyroidectomy or initial Tg <0.2 µg/L: TSH target 0.5–2 mU/mL
Total thyroidectomy and initial Tg ≥0.2 µg/L: TSH target 0.1–0.5 mU/L |
FTC with all of the following:
- Well-differentiated and minimal vascular invasion (<4 foci)
|
Intermediate
(5–20%) |
PTC with at least one of the following:
- Size >4 cm, microscopic tumour invasion into perithyroidal tissues, vascular invasion, aggressive histology subtypes
- Macroscopic regional lymph node metastases
|
TSH target:
0.1–0.5 mU/L |
FTC with:
- Locoregional lymph node metastases
|
High
(>20%) |
PTC with at least one of the following:
- Macroscopic tumour invasion of perithyroidal tissues, poorly differentiated subtype
- Large locoregional metastases or lymph nodes with extranodal extension
- Distant metastases
- Incomplete tumour resection
|
TSH target:
<0.1 mU/L |
FTC with at least one of the following:
- Widely invasive or extensive vascular invasion
- Incomplete resection
- Distant metastases
|
*TSH suppression targets are age and comorbidity dependent (refer to main text).
FTC, follicular thyroid carcinoma; PTC, papillary thyroid carcinoma; Tg, thyroglobulin; TSH, thyroid-stimulating hormone |
These factors, along with the patient’s biochemical and structural response to initial treatment and whether radioactive iodine (RAI) ablation was performed, are used to determine the recommended surveillance requirements and targets for TSH suppression. This will also inform when the patient is suitable for shared care follow-up with their GP.
Surveillance
The principal modalities used for surveillance are clinical assessment, thyroid function tests, biochemical markers of tumour recurrence and ultrasonography to assess for locoregional structural recurrence. Less than 10% of patients with DTC experience distant metastases, and half of these are identified at diagnosis.5 Lungs and bone are the most prevalent sites and risk factors include aggressive histological subtypes, vascular invasion, advanced primary tumours and bulky nodal disease.4
A consultation for thyroid cancer surveillance involves a screen for symptoms of recurrent disease in the thyroid bed, cervical lymph nodes and upper aerodigestive tract (eg dysphonia, dysphagia, pain, haemoptysis). Symptoms of hypo/hyperthyroidism should also be elicited and correlated with thyroid function tests. The anterior and lateral neck should be palpated to assess for disease in the thyroid bed (and contralateral lobe in case of hemithyroidectomy) and both central and lateral cervical lymph node compartments.
The ATA DTC guidelines4 provide surveillance recommendations based on variable levels of evidence and ultimately the follow-up algorithm is at the discretion of the thyroid specialist, taking into account both patient and disease factors. Continued risk stratification should be undertaken throughout the surveillance period to provide individualised management recommendations to the patient.
It is important for the thyroid specialist to communicate to the GP the expected frequency of investigations and follow-up, along with TSH targets and guidelines for when to re-refer. This cohort of patients with low- and-intermediate risk DTC who achieve an excellent response to initial treatment should be surveilled six monthly for the first two years and then 12 monthly thereafter (example in Table 2).4 Patients who achieve an incomplete or indeterminate response to treatment should continue follow-up with their thyroid specialist.
| Table 2. Example of follow-up schedule for DTC patients with an excellent response to initial treatment* |
| Risk of recurrence |
Initial treatment |
Example of long-term follow-up schedule for patients with an excellent response to initial treatment* |
| Low risk |
Hemithyroidectomy |
Likely suitable for GP follow-up:
Clinical history and examination six monthly for two years, then 12 monthly
Periodic ultrasonography of the neck
(eg 12–24 monthly); contralateral lobe nodules should be monitored/investigated in accordance with ATA guidelines |
| Low/intermediate risk |
Total thyroidectomy +/– RAI |
Likely suitable for GP follow up:
Clinical history and examination six monthly for two years, then 12 monthly
TFTs 12 monthly (TSH target 0.5–2 mU/mL)
Tg and TgAb levels 12 monthly
Periodic ultrasonography of the neck
(eg 12–24 monthly) |
| High risk |
Total thyroidectomy + RAI |
May be suitable for shared care or GP follow-up after 2–5 years:
Clinical history and examination 6–12 monthly for five years, then 12 monthly
TFTs 12 monthly (consider suppression TSH target 0.1–0.5 mU/mL for five years;† thereafter 0.5–2 mU/mL if no concerns for recurrence)
Tg and TgAb levels 6–12 monthly
Periodic ultrasonography of the neck
(eg 6–12 monthly) |
*Only for patients who have had an excellent biochemical and structural response to initial treatment. Patients who have an incomplete or intermediate response to treatment should continue management and follow-up with their thyroid specialist (refer to main text).
†TSH suppression targets are age and comorbidity dependent (refer to main text)
ATA, American Thyroid Association; RAI, radioactive iodine; TFTs, thyroid function tests; Tg, thyroglobulin; TgAb, anti-thyroglobulin antibody; TSH, thyroid-stimulating hormone |
There is no ATA recommendation on duration of surveillance.4 While the majority of recurrences occur within the first five years,7 late recurrences are well documented, necessitating long-term follow-up.4,8,9
Biochemical surveillance
Serial monitoring of serum thyroglobulin (Tg) and anti-thyroglobulin antibodies (TgAbs) is an important postoperative tool to assess for residual and recurrent disease in DTC.4 Ultrasensitive assays allow testing while on levothyroxine supplementation; however, consistent measuring at the same pathology provider is required to ensure accurate results.
Thyroglobulin is a protein made by thyroid follicular cells and is a sensitive marker for the presence of thyroid tissue in the body.10 It does not distinguish between benign and malignant tissue and can be elevated in most thyroid diseases, so it is not recommended to be tested preoperatively. Thyroglobulin is influenced by both the serum TSH and TgAb levels and should be considered in the context of these values.4 After total thyroidectomy and adjuvant RAI, Tg levels should become and remain undetectable. Remnant normal thyroid tissue may cause a low level of serum Tg to persist postoperatively in some patients treated with total thyroidectomy without RAI, and is expected in patients treated with hemithyroidectomy alone. While it can be considered,4 the current evidence does not support using Tg as a biomarker for recurrence in hemithyroidectomy.11
Serum TgAbs are present in approximately 25% of patients with thyroid cancer, especially in the setting of Hashimoto thyroiditis.4 The presence of TgAbs can interfere with the serum Tg level, and they should be tested together to allow accurate interpretation.4 Positive TgAbs frequently cause falsely low serum Tg levels, but can conversely cause false elevated readings with certain assays.
Patients should have undetectable or down-trending Tg and TgAbs after initial treatment. Newly elevated or rising Tg or TgAb titres raise the likelihood of persistent or recurrent disease.4
Structural surveillance
Ultrasonography of the neck is a highly sensitive tool for detecting structural recurrence in the thyroid bed, contralateral lobe (in hemithyroidectomy) and metastases to cervical nodal compartments.4 Whereas most recurrences of PTC are confined to the neck, FTC typically metastasises distantly (to lungs and bone) and rarely involves the cervical lymph nodes.10 Therefore in FTC, ultrasonography serves mainly to exclude residual/recurrent disease of the thyroid bed. The ATA recommends ultrasonography 6–12 months after treatment to measure the initial response, then ‘periodically’ depending on recurrence risk, Tg and clinical suspicion.4
Ultrasonography findings should be considered in the context of the biochemical and clinical picture. Sonographically suspicious lymph nodes ≥8–10 mm in smallest diameter should be considered for fine-needle aspiration biopsy.4 Smaller and benign appearing nodes can often be monitored with serial examinations in the first instance.4 In patients treated with hemithyroidectomy, thyroid nodules in the contralateral lobe should be monitored for growth or suspicious features and biopsied in accordance with ATA guidelines for thyroid nodules.4
TSH suppression
Lifelong thyroid hormone supplementation (levothyroxine) is required following total and completion thyroidectomy to prevent symptomatic hypothyroidism. Following surgery for thyroid cancer, supraphysiological dosing of levothyroxine is used to reduce TSH to below-normal levels via the pituitary feedback loop. TSH has a trophic effect on DTC cells, upregulating cell growth and production of proteins including Tg. By controlling the TSH-driven proliferation of DTC, levothyroxine suppressive therapy is thought to decrease the risk of disease recurrence and progression postoperatively.12
Only 10–15% of patients with normal preoperative thyroid function who undergo hemithyroidectomy will develop hypothyroidism requiring levothyroxine supplementation.4 There is little evidence to guide TSH targets or the use of the levothyroxine for TSH suppression alone in patients who underwent hemithyroidectomy.
Different guidelines recommend varying TSH targets based on their risk of recurrence and response to initial treatment, and decisions will be made by the treating thyroid specialist.4,5 Initial TSH suppression is recommended for intermediate and high-risk DTC and for cases of low-risk DTC with a detectable serum Tg measured 6–12 weeks after total thyroidectomy (Table 1). For long-term follow-up, all patients at low- and intermediate-risk patients with an excellent response to treatment (no structural or biochemical evidence of residual disease) allow TSH target levels in the low end of the normal range (0.5–2 mU/mL; Table 2). Those with high risk of recurrence or incomplete or indeterminate response to initial treatment are often recommended continued TSH suppression targets.4
Benefits of suppression must be weighed against the risk of complications from subclinical hyperthyroidism as a result of supraphysiological levothyroxine supplementation. These include exacerbation of ischaemic heart disease and atrial fibrillation, as well as osteoporosis, especially in postmenopausal women.13 A patient’s age, comorbidities and risk of recurrence should be taken into account when determining the degree of TSH suppression.4 Maintaining appropriate thyroxine replacement dosing is one of the challenges of long-term follow-up and should be reviewed at least yearly.
When to refer
Referral to a thyroid cancer specialist for further investigation and management (Box 1) is recommended for patients with suspected recurrent disease based on clinical findings, ultrasonography or blood tests.5 Patients presenting with upper aerodigestive tract symptoms such as voice change or difficulty swallowing should be referred for endoscopic assessment. Late recurrences do occur and should be considered in any patient with thyroid cancer presenting with these symptoms, irrespective of time since diagnosis.
| Box 1. Red flags for thyroid cancer recurrence |
New aerodigestive tract symptoms (eg dysphonia, dysphagia, haemoptysis)
Newly elevated or rising thyroglobulin or anti-thyroglobulin antibody levels
Enlarging or suspicious masses in the contralateral thyroid lobe, thyroid bed or cervical lymph node chain
Systemic symptoms of malignancy and metastatic disease (especially lungs and bone) |
Recent ultrasonography and blood tests are essential as part of the referral. Further investigation with fine-needle aspiration of suspicious lymph nodes or nodules is helpful but not essential and should not delay referral.
Patients with difficult-to-control thyroid function or concerns for adverse events related to supraphysiological levothyroxine dosing should be referred to an endocrinologist. Allied health disciplines play a valuable role in treatment-related dysfunction such as impaired swallow and voice, lymphoedema management and neck stiffness.
Conclusion
Many patients with DTCs can receive part of their follow-up care in general practice, the process being guided by surveillance algorithms and published guidelines.