Infertility, defined as the inability to conceive within one year without using birth control methods,1 affects up to 15% of couples.2 Couples may be further categorised into those with primary infertility, where clinical pregnancy has never been achieved, or secondary infertility, where couples have been able to achieve a clinical pregnancy at least once before with the same or different partner.1 A contributing male factor is responsible in up to 50% of cases, and the male partner should therefore undergo assessment by a doctor trained in male reproduction.3–5 Of concern, however, is that surveys suggest that the male was not evaluated in 18–27% of couples with infertility.6 With the aid of technological advances – especially in the fields of microsurgery, endoscopy, laparoscopy, assisted reproductive technology (ART) and now robotics – the surgical treatment of male infertility has expanded, with more tailored, safer and evidence-based options available. This article will outline the role of surgery in treatment of male infertility, which can assist general practitioners (GPs) in counselling their patients regarding management options. The surgical treatment of male infertility can be divided into four broad categories: surgery for diagnostic purposes, surgery to improve semen parameters, surgery to improve sperm delivery and surgery to retrieve sperm for in vitro fertilisation (IVF).
Surgery for diagnostic purposes
A goal of the workup of a patient with azoospermia is the diagnosis of either an obstructive or non-obstructive cause. Should the history, physical examination or biochemical testing or imaging remain inconclusive, the histological or cytological presence or absence of spermatozoa within the testis is currently the only way to differentiate between an obstructive cause (where ample sperm will be found in the testis as spermiogenesis is normal) or a non-obstructive cause (no sperm readily found because of a spermiogenesis problem).7 Although considered diagnostic, a biopsy is potentially therapeutic, whereby if mature spermatozoa are identified they may be used fresh or cryopreserved for use in ART. The disadvantages of this approach for sperm retrieval include the fact that in the setting of obstruction post vasectomy, reversal is more cost effective than ART, and in the setting of non-obstructive azoospermia, testicular heterogeneity may ultimately require multiple biopsies, which can lead to fibrosis and make subsequent interventions difficult. For these reasons, the role of diagnostic biopsies has now greatly diminished, and they are no longer routinely recommended.8,9
If a biopsy is performed, however, three methods may be used to obtain testicular samples sufficient for diagnostic purposes: either an open testis biopsy, percutaneous needle biopsy with a core needle or fine-needle aspiration. Each method may be performed under local, regional or general anaesthesia, depending on surgeon and patient preference.
Surgery to improve semen parameters
For patients with primary infertility, the presence of a varicocele is much more common, occurring in up to 40% of men in comparison to 15% of the general population; in men with secondary infertility, this may be up to 81%.10,11 The specific semen parameter affected most by the presence of a varicocele is concentration, followed by motility and morphology, with further evidence to suggest that serum testosterone levels are also adversely affected.12 Numerous theories for the exact cause of impairment of spermatogenesis are postulated and, although incompletely understood, generally cite alteration in the testicular microenvironment by temperature insult, oxidative stress from reactive oxygen species, reflux of toxic metabolites or alteration in normal acid–base balance.11 One measurable outcome of this altered microenvironment is sperm DNA fragmentation (the accumulation of single- and double-strand DNA breaks). Evidence suggests that varicocele repair improves DNA fragmentation and ultimately successful pregnancy rates in men with not only oligospermia, but even azoospermia, as well as couples where there has been failed implantation, embryogenesis or recurrent pregnancy loss using ART.13–15 Therefore, patients should be aware that removing a varicocele may improve ART outcomes.
Clinically, varicoceles can be usefully classified into:9,16
- subclinical – not palpable or visible at rest or during Valsalva manoeuvre, but shown on Doppler ultrasound imaging
- Grade 1 – palpable when standing and performing a Valsalva manoeuvre
- Grade 2 – palpable when standing without a Valsalva manoeuvre
- Grade 3 – visible and palpable when standing.
Poorer semen parameters are associated with higher grades.17 Meta-analysis of varicocele repair in men with subclinical or non-palpable varicoceles concluded that varicocele repair is ineffective in increasing the chance of spontaneous pregnancy.18 In contrast, meta-analysis of surgical varicocele repair in men with clinical varicocele and impaired semen quality has concluded that varicocele repair is effective and results in higher spontaneous pregnancy rates (odds ratio 4.15; 95% confidence interval: 2.31, 7.45; P <0.001).19 Guidelines therefore recommend that varicocelectomy is indicated in infertile couples in which the male has a clinical or palpable varicocele, abnormal semen parameters and otherwise unexplained infertility while the female partner has good ovarian reserve.9 In an adolescent, prophylactic treatment is indicated where there is a documented testicular size differential of >20% confirmed by serial clinical or Doppler ultrasound examination and/or abnormal semen analysis.20
Multiple options are available for varicocele repair and can be classified into radiographic (anterograde or retrograde sclerotherapy, retrograde embolisation), open (scrotal, inguinal, retroperitoneal, microsurgical inguinal or subinguinal) or laparoscopic or robot assisted.9 The principles of successful repair remain the same in all approaches, namely to ligate or occlude the veins contributing to varices while preserving adequate venous drainage as well as the arteries, vas and lymphatics. The most important complications are recurrence, hydrocele formation and testicular atrophy.21 While all options are able to be performed as a day procedure, microsurgical varicocelectomy is considered to be the most effective, with the lowest complication and recurrence rates based on case series, with no randomised controlled trials available for direct comparison.22,23 In the absence of microsurgical training, all options are still considered viable, albeit with higher recurrence and hydrocele rates, notwithstanding specific potential complications unique to each modality (Table 1). The clinician must evaluate patient factors (eg previous inguinal or intraperitoneal surgery, contrast allergy, anaesthetic comorbidities), clinician factors (availability of expertise) and external factors (eg cost) when selecting a method of repair. In the adolescent population, a majority of paediatric surgeons prefer a laparoscopic approach.24
| Table 1. Varicocele treatment modalities: Recurrence rates and complications9 |
| Treatment |
Recurrence/
persistence (%) |
Overall complications |
Specific complications |
| Anterograde sclerotherapy |
5–9 |
Hydrocele (5.5%), haematoma, infection, scrotal pain, testicular atrophy, epididymitis |
Technical failure (1–9%), left flank erythema |
| Retrograde sclerotherapy |
6–9.8 |
Hydrocele (3.3%), wound infection, scrotal pain |
Technical failure (6–7.5%), adverse reaction to contrast medium, flank pain, persistent thrombophlebitis, venous perforation |
Retrograde
embolisation |
3–11 |
Hydrocele (10%), haematoma, wound infection |
Technical failure (7–27%), thrombophlebitis, reaction to contrast medium, misplacement or migration of coils (to femoral vein or right atrium), retroperitoneal haemorrhage, fibrosis, ureteric obstruction, venous perforation |
| Inguinal approach |
2.6–13 |
Hydrocele (7.3%), testicular atrophy, epididymo-orchitis, wound complications |
Postoperative pain due to incision of external oblique fascia, genitofemoral nerve injury |
| Open retroperitoneal high ligation |
15–29 |
Hydrocele (5–10%), testicular atrophy, scrotal oedema |
External spermatic vein ligation failure |
Microsurgical inguinal
or subinguinal |
0.4 |
Hydrocele (0.44%), scrotal haematoma |
|
| Laparoscopy |
3–6 |
Hydrocele (7–43%), epididymitis, wound infection, testicular atrophy due to injury to testicular artery, bleeding |
External spermatic vein ligation failure; intestinal, vascular and nerve injury; pulmonary embolism; pneumoscrotum; peritonitis; postoperative pain to right shoulder due to diaphragmatic stretching during pneumoperitoneum |
Surgery to improve sperm delivery
Approximately 25,000–30,000 vasectomies are performed annually in Australia (median 110 per 100,000 population in 2017–21).25 The number of vasectomy reversals is only a fraction of this number, with a median of 811 unilateral procedures performed annually from January 2017 to December 2021.26 International literature suggests that 2–6% of men who have undergone a vasectomy will ultimately request a reversal.27,28 A considered evaluation must be made should a man request restoration of fertility following vasectomy; that is, to either opt for vasectomy reversal or IVF and intracytoplasmic sperm injection (ICSI). Factors to be considered include the age of the female partner, the number of children desired, desire for natural conception, the interval since vasectomy and cost.29 Sperm commonly returns to the ejaculate within a few weeks but may take up to six months to two years.30 These authors do not recommend routine antisperm antibodies prior to undergoing vasectomy reversal as there is no correlation between antisperm antibodies and post-reversal fertility.31
Successful vasovasostomy reversal rates decline the longer the interval time since vasectomy, especially after 10 years, which may require a more technically difficult vasoepididymostomy to overcome the secondary epididymal obstruction – the incidence of which increases with increasing time since vasectomy.32 However, collated Australian data suggest that even with an interval of more than 10 years, approximately 82% of patients can have sperm restored to the ejaculate.33 The advantages of vasectomy reversal include treatment of the affected man (avoiding the not insignificant risks of IVF in the female partner),34 natural conception, the ability to father more than one child and the need for only one procedure, which is generally more cost effective when compared with IVF/ICSI.29,35,36 Microsurgical vasovasostomy (Figure 1) or vasoepididymostomy has long been considered the standard of care, with consistently superior results when compared with non-microsurgical techniques.9,28,37–39
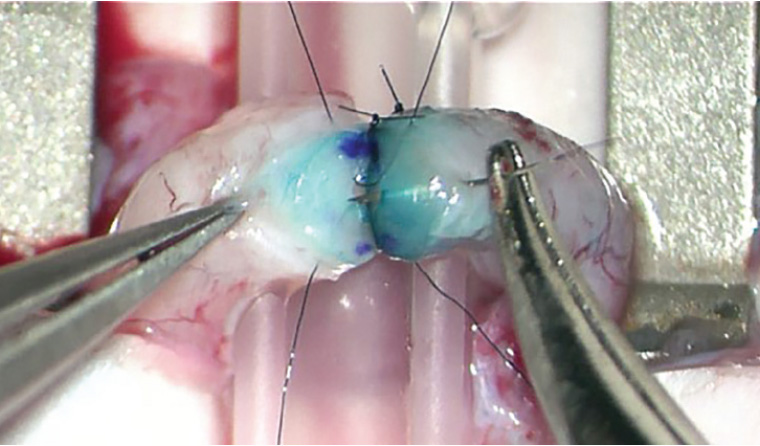
Figure 1. Microsurgical vasovasostomy. Under an operating microscope, here a modified two-layer anastomosis is performed using 9-0 nylon. The cut edges have been stained with methylene blue and marked with ink microdots to aid precise apposition.
Intra-operative photograph supplied by Dr Darren J Katz
Ejaculatory duct obstruction (EDO) accounts for 1–5% of male infertility.40 Cardinal features of this condition are low ejaculate volume (<1.5 mL), semen pH <7.2 and absence of seminal fructose. Underlying aetiologies may be congenital (cysts, diverticulae, atresia), acquired (calculi, inflammatory, iatrogenic) or functional.40,41 The traditional treatment is transurethral resection of ejaculatory ducts (TURED) using a 24 Fr resectoscope and electrocautery loop.42 Novel treatment by way of transurethral seminal vesiculoscopy using 6 Fr ureteroscopes allows for simultaneous diagnosis and treatment and minimises the potential side effects of TURED, which are epididymitis, watery ejaculate, urinary incontinence and rectal injury.43–45
Transrectal ultrasonography and/or magnetic resonance imaging has now largely supplanted vasography as a means to diagnose EDO.46 An anteroposterior measurement of the seminal vesicle >15 mm is highly suggestive of EDO.46,47 Vasography, being an open, invasive procedure, should only be performed at the time of planned reconstruction and is rarely used as a pure diagnostic procedure.48 It may be performed using a 30-gauge lymphangiogram needle or via a hemivasotomy requiring microsurgery skills. The presence of sperm and the location of obstruction guides subsequent reconstruction.48
Men with spinal cord injuries comprise a special subset of patients with anejaculatory infertility, particularly as this population is typically between the ages of 16 and 45 years.49 The first-line treatment is with penile vibratory stimulation, using an optimal vibration amplitude of 2.5 mm with an approved device applied to the glans penis.50 Men with injuries at the level of the T10 vertebra or higher may achieve >80% successful ejaculation.48,51 Electroejaculation, usually performed under general anaesthetic, is usually successful in achieving seminal emission in all causes of anejaculation.51 Treatment with penile vibratory stimulation or electroejaculation affords the couple the opportunity for pregnancy by home intravaginal insemination, intrauterine insemination or ART.51
Surgery to retrieve sperm for IVF
The ability to achieve IVF using a single sperm with ICSI, even with sperm with limited fertilising capacity direct from the testis, enabled the development of methods to retrieve sperm in men who have azoospermia, a condition that affects 1% of all men and 10–20% of males who present with infertility.7 Sperm may be retrieved from the vas, epididymis or testis either by a percutaneous approach (aspiration or biopsy) or using an open technique (with or without an operating microscope). The choice of technique depends on the clinical presentation (especially if it is obstructive or non-obstructive azoospermia) and the skillset of the surgeon. From the testis, techniques include testicular sperm aspiration (TESA), conventional testicular sperm extraction (cTESE) and microdissection testicular sperm extraction (microTESE). From the epididymis, techniques include percutaneous epididymal sperm aspiration (PESA) and microsurgical epididymal sperm aspiration (MESA).
Obstructive azoospermia
Obstructive azoospermia accounts for 40% of cases of azoospermia.7 Spermatogenesis is generally not affected in obstructive azoospermia, and if reconstruction is not an option, or there are significant female factors that mean ART is indicated, sperm may be retrieved from the testis or epididymis using TESA, PESA or MESA. The best technique to select is not entirely clear: both the American Urological Association (AUA) 2021 and European Association of Urology (EAU) 2022 guidelines advise that regardless of the source of sperm retrieved, or the cause of obstruction, the outcome of IVF with ICSI in obstructive azoospermia is similar.8,9 The American Society for Reproductive Medicine 2019 guidelines suggest that MESA (Figures 2A and B) yields the best live birth rates in cases of obstructive azoospermia.52,53 Each technique has its advantages and disadvantages. Ultimately the choice is dependent on local facility and surgeon experience (Table 2).52
| Table 2. Surgical techniques to obtain sperm for in vitro fertilisation for obstructive azoospermia |
| Site |
Procedure |
Technique |
Advantages |
Disadvantages |
| Epididymis |
Percutaneous epididymal sperm aspiration (PESA) |
A 21-gauge needle attached to a 10 mL syringe containing nutrient medium is used to puncture the epididymis under local anaesthetic. |
- Local anaesthetic
- Simple technique
- Epididymal sperm more mature and motile than testicular sperm
- No microsurgical training required
|
- Blind nature of procedure
- Higher risk of vessel injury, spermatocoele and fibrosis
- Sperm retrieval rate 80%
- Insufficient sperm numbers for cryopreservation
|
| Microsurgical epididymal sperm aspiration (MESA) |
Under general anaesthetic, the scrotum is opened and testis exposed. Enlarged epididymal tubules are selected under an operating microscope and overlying tunica excised. The tubule is punctured with a microknife and fluid aspirated with a 24-gauge angiocatheter or micropipette. Puncture sites are subsequently sealed with bipolar cautery. |
- Sperm retrieval
rate 90%73
- Large numbers of sperm obtained; allows for cryopreservation and flexibility with in vitro fertilisation timing
|
- General anaesthetic required
- Requires microsurgical training
|
| Testis |
Testicular sperm aspiration (TESA) |
An 18- to 21-gauge needle is used to puncture the testis, under local anaesthetic, and moved back and forth multiple times inside the testis with negative pressure exerted on a 20 mL syringe. The contents are then expelled into a tube containing nutrient medium. |
- Local anaesthetic
- Simple, rapid technique
- No microsurgical training required
|
- Lowest sperm yield, numbers generally insufficient for cryopreservation (unless multiple passes of the needle made)
|
Non-obstructive azoospermia
The majority of non-obstructive azoospermia cases remain idiopathic, with a subset attributable to an underlying cause, either genetic or acquired. Spermatogenic failure is the hallmark of non-obstructive azoospermia. Despite this, well over 50% of men will harbour sperm within the testes, which, if found and using ART, can lead to live births in up to 28% of couples.54 For example, sperm may still be found using microTESE in up to 47% of cases where a biopsy showed Sertoli-only cells, a condition formerly thought to be a strong negative predictor for successful sperm retrieval.55 In non-obstructive azoospermia, retrieval of sperm from the testes is the only option. Both the AUA 2021 and EAU 2022 guidelines recommend the use of microTESE over cTESE given the superior sperm retrieval rates, lower risk of causing iatrogenic hypogonadism, lower volume of testicular tissue removed and lower complication rates of haematoma and fibrosis (Table 3).8,9 The fundamental technique in microTESE is to find and harvest seminiferous tubules containing sperm, which are generally larger and more opaque (Figure 2E) when seen under an operating microscope with 15–20× magnification.56 The technique of cTESE is essentially the same as testicular biopsy except that the testis is delivered through the scrotum and multiple sites are sampled, without using an operating microscope, with the aim of removing 50 mg of tissue at each biopsy site (Figures 2C and D).
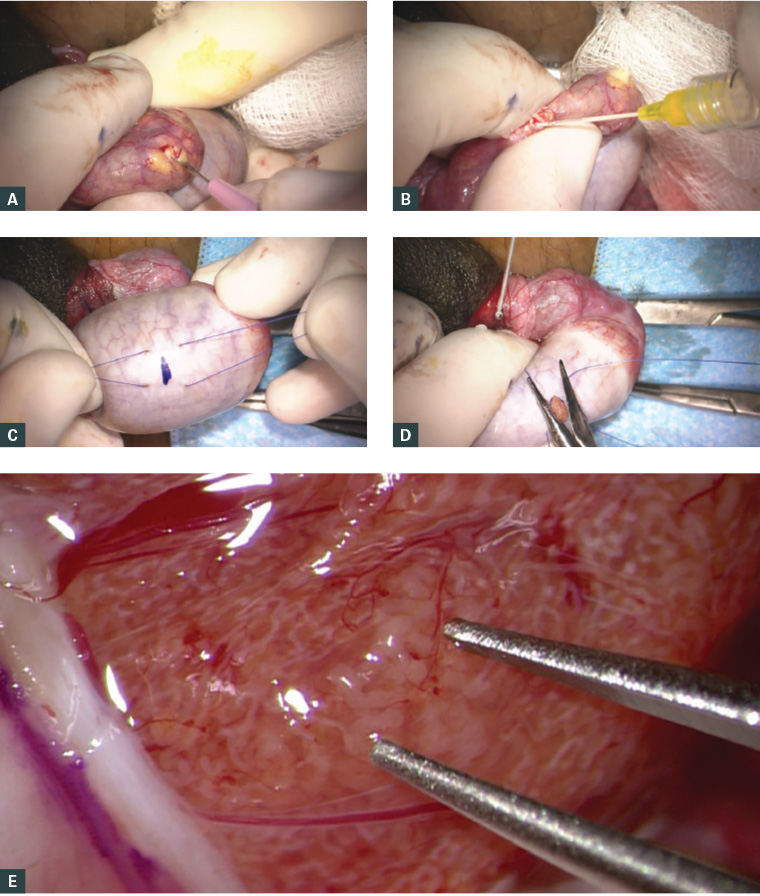
Figure 2. Surgical sperm retrieval methods for in vitro fertilisation
a. Microsurgical epididymal sperm aspiration (MESA): dilated epididymal tubule about to be incised with a microknife; b. Epididymal fluid aspirated using a 24-gauge cannula for subsequent microscopic evaluation for motile sperm; c. Conventional testicular sperm extraction (cTESE): 5-0 prolene stay sutures placed either side of the site marked for incision of the tunica albuginea; d. Extruded seminiferous tubules about to be cut for sampling and delivery. The stay sutures are used to close the defect; e. Microdissection testicular sperm extraction (microTESE): under 15–20× magnification, a focus of dilated seminiferous tubules can be identified between the tips of the micro forceps that is likely to harbour sperm.
Intra-operative photographs supplied by Dr Darren J Katz
| Table 3. Surgical techniques to obtain sperm for in vitro fertilisation for non-obstructive azoospermia |
| Site |
Procedure |
Technique |
Advantages |
Disadvantages |
| Testis |
Conventional testicular sperm extraction (cTESE) |
Under general anaesthetic the scrotum is opened in the midline and tunica albuginea of the testis exposed. Expressed seminiferous tubules are extracted through 4–5 mm incisions made in the tunica albuginea, aiming for approximately 50 mg of tissue. Multiple incision sites may be required. Incisions are closed with absorbable or permanent sutures. |
- No microsurgical training required
- Overall sperm retrieval rate 16.7–45%74
- Superior sperm retrieval rates when compared with TESA (2× higher)75
|
- General anaesthetic required
- Multiple sites may be required to obtain sperm
- Higher risk of fibrosis, haematoma formation, hypogonadism when compared with microTESE
|
| Microdissection testicular sperm extraction (microTESE) |
Under general anaesthetic the testis is delivered and bivalved equatorially. Under an operating microscope, a systematic search for enlarged tubules, more likely to contain sperm, is performed; these are extracted with jeweller’s forceps. Tubules are then morcellated in nutrient medium and inspected under 400× magnification for the presence of sperm. The tunica is then closed with running 5-0 prolene suture. |
- Overall sperm retrieval rate 42.9–63%74
- Superior sperm retrieval rates when compared with cTESE (1.5× higher)75
|
- General anaesthetic required
- Requires microsurgical training
- Longer operating time
|
New technologies
The turn of the millennium saw the widespread adoption of the da Vinci robot in the field of urology.57,58 The first proof of concept of robot-assisted human vasectomy reversal was in 2004,59 varicocele repair in 200860 and microTESE in 2013.61 Theoretical advantages include elimination of physiological tremor, increased ergonomics, stable magnification, reduced learning curve and removal of the need for a skilled microsurgery assistant.62 There is a clear, albeit rare, advantage in the setting of repair of intraperitoneal vasal obstruction.63,64 Urology trainees, who have an early exposure to robotics because of its widespread use, may have an advantage over other surgical specialties with the application of robotic technologies to surgical procedures. Disadvantages, however, include the lack of haptic feedback, the lack of a range of robotic microsurgical instruments, the fact that the robot was not primarily designed for microsurgery, inferior optics and significant cost.62 It remains to be seen whether robot-assisted devices will be routinely used in the fertility field, with effects on the outcomes, costs, training and surgeon benefits yet to be conclusively determined. As suturing is the most technically challenging and time-consuming step in vasectomy reversal,29 evaluation of sutureless techniques such as laser welding,65,66 fibrin glues,67 biomechanical sealants68 and microclips69 is ongoing.
Adjuncts to aid identification of sperm-containing tubules during microTESE – such as Raman spectroscopy,70 multiphoton tomography71 and probe-based laser confocal endomicroscopy72 – are in their infancy but have the promising advantage of reducing the not insignificant operative time and increasing sperm retrieval rates.
Conclusion
The armamentarium of techniques now available to the operating urological surgeon for the treatment of male infertility has expanded rapidly over the past decades and continues to evolve. Refinements in technique and a team approach with the GP, clinical and laboratory-based andrologists and embryologists have allowed couples to successfully conceive where formerly there was limited hope. Knowledge of current and evolving treatments in the management of male infertility can assist GPs in managing this important patient group.
Key points
- A contributing male factor is responsible in up to 50% of couples with infertility; therefore, men need to be equally evaluated in the assessment of infertility.
- Surgical management to treat the various forms of male infertility is best undertaken by a urologist trained in microsurgery.
- Clinical varicocele is a common and reversible cause for male infertility.
- Microsurgical vasectomy reversal is a cost-effective treatment option when compared with ART.
- MicroTESE is the preferred technique for sperm retrieval in the setting of non-obstructive azoospermia.