Aortic stenosis (AS) is the most common form of valvular disease in Australia and affects the elderly, with a growing prevalence of up to 10% in octogenarians.1,2 Degenerative AS is caused by calcium deposition on the aortic valve leaflets, with progressive narrowing of the valve orifice and a subsequent reduction in cardiac output. Once symptomatic, severe AS is associated with a poor prognosis, with mortality rates worse than for some malignancies.3–5 Patients with severe AS should be assessed for consideration of treatment by a multidisciplinary heart team. In the elderly, transcatheter aortic valve implantation (TAVI) is now the recommended treatment for severe AS in those deemed suitable for intervention.6,7 Interactions between a patient’s general practitioner (GP) and other members of the heart team are important to recognise futility of treatment, for example due to severe frailty, significant comorbidities/other major organ system compromise and/or a high preoperative risk of mortality and morbidity. This narrative describes the contemporary management of severe AS from a GP’s viewpoint.
Clinical features
Patients with AS will often have a prolonged latent period of asymptomatic disease and only develop symptoms once AS is moderate or severe.8 The most common symptoms of AS are shortness of breath on exertion, a reduction in exercise tolerance, angina and syncope.9 The frail and elderly may present with non-specific symptoms, such as fatigue and an inability to undertake activities of daily living.10 Often a murmur suggestive of AS will be incidentally detected on routine examination before a definitive diagnosis is achieved with echocardiography.9 Physical examination findings suggestive of severe AS include a low volume and slow to rise carotid pulse, ejection systolic murmur, which is loudest in the right second intercostal space and radiates to the carotids, and a single or paradoxically split-second heart sound (S2).11 In elderly patients with degenerative AS, the murmur of AS may radiate towards the apex with a high-pitched quality; this is known as the Gallavardin phenomenon and can be mistaken for mitral regurgitation.12
Evaluation and investigation
Patients with suspected severe AS should undergo transthoracic echocardiography (TTE). TTE is used to assess the structure and haemodynamics of the valve, left ventricular size and function and other structural cardiac disease.13 Following TTE, AS is categorised according to severity using aortic valve area (AVA), the peak velocity of blood flow through the valve and the median pressure gradient across the valve (Table 1; Figure 1).14 If AS is confirmed on echocardiography, management and follow up are determined by disease severity. For patients with mild AS, two-yearly serial TTE may be appropriate and should be reduced to yearly TTE for those with moderate AS. Patients with symptomatic, severe AS should be considered for valve intervention.
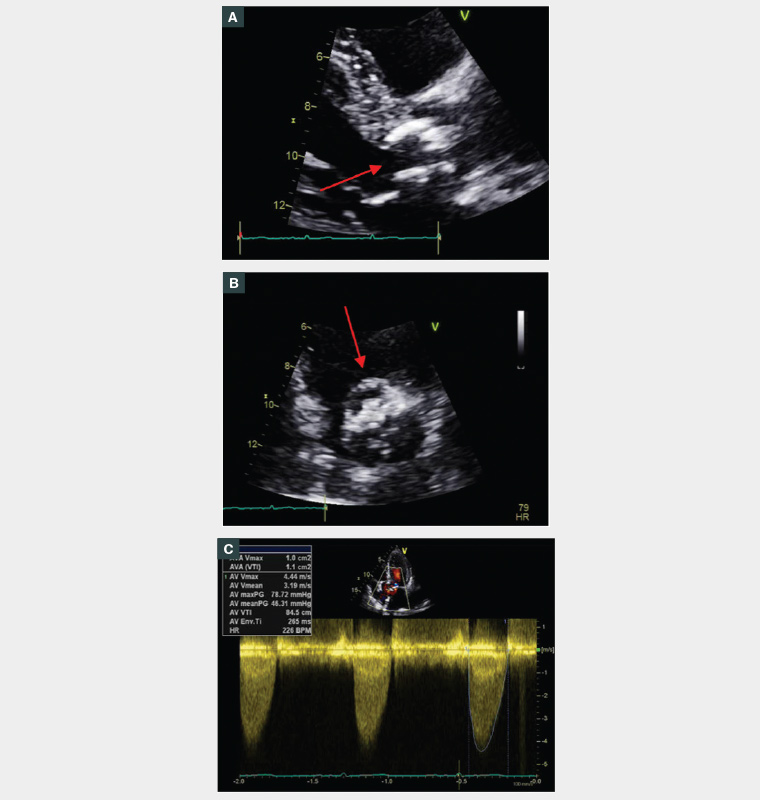
Figure 1. Transthoracic echocardiogram findings of severe aortic stenosis (AS).
A. Parasternal long axis view of a heavily calcified aortic valve (red); B. Short-axis view of a heavily calcified aortic valve (red) with minimal leaflet excursion and a reduced orifice area; and C. Typical haemodynamic profile of a continuous wave Doppler signal of severe AS.
| Table 1. Grading of aortic stenosis severity on transthoracic echocardiography |
| |
Aortic stenosis |
| Mild |
Moderate |
Severe |
| Peak velocity (m/s) |
2.6–2.9 |
3.0–4.0 |
≥4.0 |
| AVA (cm2) |
>1.5 |
1.0–1.5 |
<1.0 |
| Mean gradient (mmHg) |
<20 |
20–40 |
≥40 |
| Indexed AVA (cm2/m2) |
>0.85 |
0.60–0.85 |
<0.6 |
| Dimensionless index |
>0.50 |
0.25–0.50 |
<0.25 |
| AVA, Aortic valve area. |
Introduction to TAVI
Management options for severe AS include TAVI, surgical aortic valve replacement (SAVR) and medical (palliative) therapy. SAVR has previously been the gold standard treatment for severe AS, but many elderly patients were frequently considered unsuitable for SAVR because of their advanced age, coexisting medical conditions and functional status.15,16 Historically, in Australia, up to one-third of patients with severe AS were not offered SAVR due to prohibitive risk.15 TAVI was initially developed for the elderly, frail or those with complex comorbidities. TAVI avoids the need for sternotomy and cardiopulmonary bypass and is performed in the cardiac catheterisation laboratory, without the need for a general anaesthetic.8 Using a percutaneous approach, a sheath is placed in the femoral artery and a valve is advanced to the aortic annulus via a guidewire (Figure 2A). A period of rapid ventricular pacing allows for valve deployment (Figure 2B,C), after which the valve leaflets work immediately (Figure 2D).8
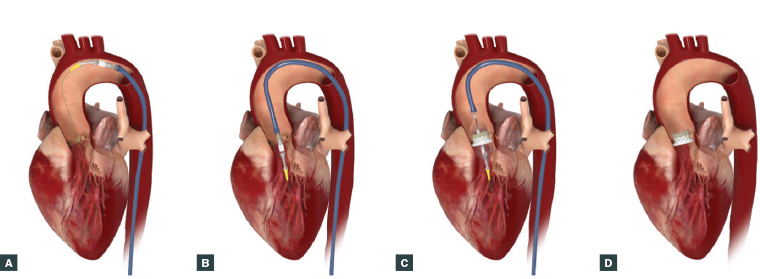
Figure 2. Procedural steps for the transcatheter aortic valve implantation (TAVI) procedure.
A. A guidewire is advanced to the aortic annulus via the femoral artery and aorta. B. The valve delivery sheath is passed over the guidewire. C. The aortic valve is balloon dilated and the valve is positioned during a period of rapid ventricular pacing. D. After successful valve implantation, the valve delivery system is removed.
Images provided by and reproduced with the permission of Edwards Lifesciences Corporation (North Ryde, NSW, Australia).
Aortic valve intervention is recommended in patients with symptomatic severe AS or asymptomatic severe AS with impaired left ventricular function (due to no other known cause) and a life expectancy of over one year.6,7 In addition to baseline TTE, preprocedural testing prior to intervention includes routine blood tests, electrocardiography (ECG), coronary angiography and a dedicated TAVI cardiac computed tomography (CT) scan. The choice of intervention (or not) is determined by evaluating the risks and benefits of each procedure in the light of patient comorbidities, functional status, level of frailty and preference (Figure 3). SAVR risk is established using validated risk scores, such as the Society of Thoracic Surgeons (STS) Predicted Risk of Mortality (PROM) score and the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II), which categorise patients into low-, intermediate- and high-risk groups.17,18 An example risk assessment is presented in Table 2. In elderly patients who are at high surgical risk, randomised control trials and large registries have shown that TAVI, compared with standard medical therapy, is associated with improved survival and reductions in cardiac symptoms and rates of rehospitalisation.3,4 In this high-risk group and in adults aged over 75–80 years, current guidelines recommend that provided transfemoral access is possible, TAVI could be considered in preference to SAVR.6,7
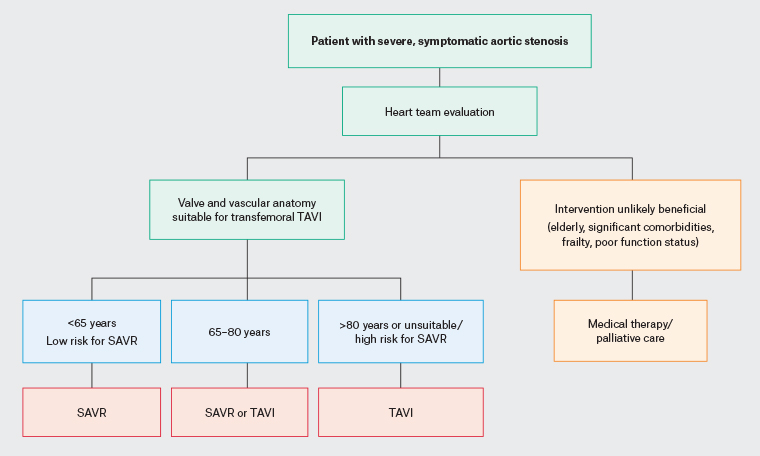
Figure 3. Management algorithm for patients with severe aortic stenosis based on the American Heart Association/American College of Cardiology and European Society of Cardiology guidelines for the management of valvular heart disease.11,12 Click here to enlarge
SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
| Table 2. Assessment and stratification of procedural risk when considering intervention for severe aortic stenosis* |
| Risk category |
Description |
| Low risk |
STS score <4% |
| No frailty |
| No comorbidities |
| No procedure-specific impediments |
| Intermediate risk |
STS score 4–8% |
| Mild frailty |
| Compromise of ≤1 major organ system |
| Minimal procedure-specific impediments |
| High risk |
STS score >8% |
| Moderate–severe frailty |
| Compromise of ≤2 major organ systems |
| Possible procedure-specific impediment |
| Prohibitive risk |
Preoperative risk of mortality and morbidity ≥50% at 1 year |
| Severe frailty |
| Compromise of ≤3 major organ systems |
| Severe procedure-specific impediments |
*Based on the American Heart Association/Americal College of Cardiology consensus statement and European Society of Cardiology guidelines for the management of valvular heart disease.6,37
STS, Society of Thoracic Surgeons. |
Heart team evaluation
The decision regarding the best management option for a patient with severe AS involves a multidisciplinary heart team.6,7 In Australia, the Medical Services Advisory Committee and Medicare Benefits Schedule mandate the use of a heart team, with auditable documentation of multidisciplinary heart team members, which may include interventional and non-interventional cardiologists, cardiothoracic surgeons, cardiac anaesthetists, the GP, geriatrician or general physician and nursing and allied health staff.19 Typically, a patient with severe AS will be referred for heart team discussion by their primary cardiologist after collaboration with the GP. The heart team considers the risks and benefits of each approach using collaborative decision making, which often occurs at multiple levels between team members. The primary cardiologist can act as an intermediary practitioner between the GP and discussions that occur in the hospital setting. Important information that can be provided by the GP includes chronological and collateral history, past medical history, assessment of functional status and cognitive function (including any prior cognitive testing, if this has been performed) and any concerns regarding specific treatment options for a particular patient. After a consensus has been reached about appropriate management options, this is conveyed to the patient and their family, who can make an informed treatment choice.
Predictors of poor outcome after TAVI
Frailty is common among patients with severe AS, affecting up to 60% of patients awaiting an intervention, and is an established predictor of poor outcomes following TAVI.20–22 Frailty assessment for elderly and high-risk patients should be routinely performed using a frailty scoring system. Several validated frailty indices exist, including the Katz Activities of Daily Living Questionnaire and Essential Frailty Toolset; these scoring systems include parameters such as physical function, cognition (evaluated using the Mini-Mental State Examination), nutrition, activities of daily living and the presence of anaemia and hypoalbuminaemia.20 In addition to frailty, comorbidities of chronic obstructive pulmonary disease, pulmonary hypertension, liver disease, advanced chronic kidney disease and Alzheimer’s disease are associated with poor outcomes after TAVI.22,23 Elderly patients with coexisting frailty and comorbidities affecting other organ systems need to be carefully considered on a case-by-case basis to determine whether the intervention will be of benefit.
Medical therapy for severe AS
For patients with symptomatic severe valvular disease, there are no medical therapies that will alter the natural history of AS. Patients who are not suitable for, or do not wish to pursue, intervention should receive medical therapy. Patients with concurrent coronary artery disease, atrial fibrillation and/or heart failure should continue guideline-directed medical therapy for these conditions.6,7 Hypertension should be treated to avoid excess afterload. Angiotensin-converting enzyme inhibitors are safe to use in severe AS and have possible beneficial myocardial effects.24 Medications that may cause symptomatic hypotension and a reduction in preload (eg vasodilators and diuretics) should be used with caution.7 Due to the poor prognosis associated with symptomatic AS and the nature of symptoms, patients should be referred early to a palliative care clinician.25,26 The introduction to palliative care should be instituted alongside medical therapy and focus on symptom management, establishing goals of care and counselling for the patient and family about disease trajectory and expected prognosis.26
Management after TAVI
Patients who undergo a successful TAVI are discharged, on average, 1–4 days after the procedure. Most patients can ambulate without restriction after TAVI and will experience an improvement in symptoms, quality of life and functional status within a short period after discharge.3,27 Delirium can be a common complication in patients after TAVI, affecting up to 25% of elderly patients.28,29 Risk factors for delirium include an alternative access approach (eg transapical), acute kidney injury, prior cognitive impairment, atrial fibrillation, stroke or transient ischaemic attack, carotid artery disease and/or peripheral artery disease.29 Although the onset of delirium typically occurs within two days after TAVI, it may be a reason for presentation to the GP in the early post-procedure period.28,30 Patients in sinus rhythm should receive antiplatelet monotherapy (aspirin or clopidogrel) lifelong for the prevention of valve thrombosis or thromboembolism. If anticoagulation is indicated for other reasons (atrial fibrillation, recurrent venous thromboembolism and/or mechanical mitral valve), this should be continued after the procedure without concurrent antiplatelet therapy.7,31 Direct oral anticoagulants are typically used rather than vitamin K antagonists, which have been associated with higher rates of major bleeding.32,33 Although the incidence of clinical thromboembolism and valve thrombosis is low after TAVI (approximately 0.7%), asymptomatic valve thrombosis has been observed in up to 15% of patients.34 The diagnosis of valve thrombosis can be challenging, and patients may present with a lack of improvement or recurrence of symptoms after TAVI. TTE should be performed as an initial investigation and, if valve thrombosis suspected, evaluated further with a cardiac 4-dimensional CT scan.35 Antibiotic prophylaxis for the prevention of bacterial endocarditis should be given prior to high-risk dental procedures (involving manipulation of the gingiva or oral mucosa), tonsillectomy/adenoidectomy or respiratory, genitourinary and gastrointestinal procedures in patients with an established infection.6 A non-driving period of four weeks is mandated for personal licence use, as per the national driver medical standards.36
Conclusion
Severe AS is a condition that commonly affects elderly patients, and once symptomatic has a poor prognosis. TAVI is now a well-established procedure with a mortality benefit that offers elderly patients a less invasive treatment option. GPs play a key role in identifying patients with AS and facilitating a swift diagnosis. The decision-making process for appropriate management for a patient with severe AS can be complex due to a multitude of factors requiring consideration (age, comorbidities, frailty, procedural logistics and risks). The heart team plays a crucial role in developing an individualised management plan and requires close collaboration between team members.
Key points
- Owing to an aging population there is a growing prevalence of severe AS in Australia.
- Severe AS without valvular intervention is associated with a poor prognosis.
- GPs are paramount in identifying patients with severe AS and facilitating timely diagnosis and treatment.
- TAVI is now a well-established procedure that enables treatment for severe AS in the elderly.
- TAVI is associated with a reduced hospital stay, expedited recovery and improved quality of life.