Case
A 58-year-old man presented to the emergency department with a 10-day history of right eye loss of vision and mucopurulent discharge. He had no history of ocular trauma or surgery. He wore soft monthly contact lenses for myopic refractive correction, which he removed once a month. His initial symptoms included mild irritation followed by increasing levels of pain and discharge, and subsequent complete loss of vision.
Clinical examination on presentation revealed no light perception vision in the right eye and 6/12 corrected vision in the left eye. Clinical photographs were taken (Figure 1A) and a non-contrast computed tomography (CT) scan of the head and orbits (Figure 1B) was undertaken to rule out abscess formation or a foreign body from unreported trauma.
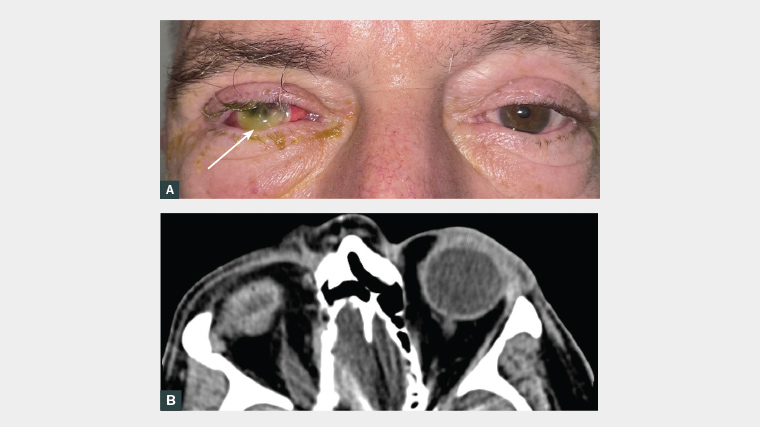
Figure 1. A. Clinical colour eye band photograph with an obvious fluorescein-stained white bulge (arrow). B. Non-contrast computed tomography scan of the head and orbits in the axial plane.
Question 1
What are the risk factors for contact lens associated keratitis (CLAK)?
Question 2
Based on the clinical eye band photograph, describe the appearance of the patient’s eye.
Question 3
What differential diagnoses need to be considered?
Question 4
What other investigations are needed to narrow the diagnosis?
Question 5
How is this condition managed?
Answer 1
In Australia, the risk of microbial keratitis is 5- to 10-fold higher in contact lens wearers than in the general population.1 The risk is higher with increased days of wear, poor hand and lens storage hygiene, sleeping with lenses, water exposure (including showering, surfing or swimming), young age, cigarette smoking, male sex, and the online purchase of lenses.2 Ocular surface disease (eg dry eyes), previous ocular surgery, topical steroid use and any underlying autoimmune disease should also be considered as additional risk factors.1,2
The most common causative organisms for severe CLAK tend to be Gram negative, in particular water-borne Pseudomonas aeruginosa.1
Answer 2
It is helpful to refresh the basic eye examination. On clinical examination, it is possible to identify eyelid redness and swelling, scleral injection and intensive fluorescein-stained cornea inferiorly. The concerning feature on this image is the fluorescein-stained white bulge (Figure 1A, arrow), which, in this circumstance, is extruded ocular content on the eye surface. If a slit lamp is at hand, it will demonstrate more clearly the extruding pigmented uveal content, as well as evidence of conjunctival injection and chemosis (swelling), corneal infiltration, corneal perforation with a flat anterior chamber and hypopyon.
Answer 3
Complete vision loss, mucopurulent discharge, flattened anterior chamber and loss of globe integrity on CT point to complete corneal melt and perforation secondary to CLAK. The delay in presentation for this patient was the main risk factor for this severe complication.3 It is important to also consider and investigate for other differential diagnoses for this presentation, namely:
- alternative causes of microbial keratitis (eg gonorrhoea)
- traumatic open globe injury
- chemical (acid or alkali) injury (note, alkali agents [eg lime and ammonia] are lipophilic and demonstrate greater penetration into the cornea than acids)
- pan-ophthalmitis due to a systemic infection (eg infective endocarditis)
- peripheral ulcerative keratitis, typically associated with systemic autoimmune disease.4–6
Answer 4
Patients suspected of having CLAK require urgent same-day ophthalmological review for baseline vision, corneal scrape (with inoculation of the corneal contents onto several slides, specialised plates and media) for culture and sensitivities, and intensive antibacterial eye drops. Acanthamoeba, and viral swabs for varicella zoster virus (VZV) and herpes simplex virus (HSV), are also usually sent. Ocular ultrasound (B-scan) is often used to assess posterior chamber involvement and identify collections. A CT scan of the orbits is helpful to exclude pan-ophthalmitis, foreign bodies and globe rupture.
Answer 5
It is important that the patient is not started on empirical antibiotics before corneal scrape and swabs. Early intensive broad-spectrum antibiotic drops are recommended as per the Therapeutic Guidelines.7 Current recommendations are ciprofloxacin 0.3% or ofloxacin 0.3% hourly (including overnight). Admission to hospital is common, with compounded fortified antibiotics preferred, specifically cefazolin 5% and gentamicin 0.9% hourly. Intravitreal biopsy and antibiotics must be administered urgently if endophthalmitis is suspected. Broad-spectrum systemic antibiotics with good ocular penetration, such as moxifloxacin, are occasionally required if there is endophthalmitis.8 A short-term course of opioid analgesia is sometimes required. Corneal transplant may be appropriate in patients to restore globe integrity or to improve vision once the corneal scar has stabilised. In severe cases, where conservative measures may contribute to the risk of pan-ophthalmitis and cerebral spread, the removal of non-viable and infected ocular contents by enucleation or evisceration may be considered for source control.9
Case continued
The patient was found to have heavy growth of P. aeruginosa in his vitreous cultures. This organism is associated with a worse prognosis than other types of microbial keratitis. Following a 24-hour trial of systemic and topical antimicrobial therapy with no change in vision, the patient elected to undergo an evisceration for definitive management, and was discharged on Day 3 postoperatively with no ocular pain or infective concerns on subsequent outpatient follow-up. Counselling to adapting to life with monocular vision and grief over loss of an eye are important issues to discuss with patients and their families. Improvements in contact lens and lens storage hygiene or the cessation of contact lens use are critical to preventing recurrent episodes. This would include avoiding direct exposure of contact lenses to water, consistent hand hygiene when dealing with contact lenses, avoiding overnight wear and increased days of wear, and managing any underlying dry ocular surface disease with lubricating eye drops. As a rule, patients with only one seeing eye are advised to avoid contact lens wear.
Key points
- P. aeruginosa is a common cause of keratitis in contact lens wearers, which may have devastating outcomes for vision and the eye.
- It is important to educate contact lens-wearing patients about the risks of microbial keratitis and how to minimise these.
- Same-day referral is key to the diagnosis and management of severe corneal infections. This can be done following a basic eye examination in the emergency department or general practice setting.
- It is important that patients are not started on empirical antibiotic therapy prior to ophthalmological review.