Case
A 59-year-old man presented to his general practitioner (GP) with a rapidly growing, sinister-appearing lump on his lower abdomen. The mass had started as a small, painless, well-circumscribed subcutaneous lump and had increased in size rapidly over the period of a week. The patient did not report any abdominal pain. However, he had developed fevers, with no significant weight loss or night sweats. His past medical history included rheumatoid arthritis (RA) on methotrexate and prednisolone for 10 years. The patient worked as an electrical engineer, never smoked and rarely drank alcohol. He was an active man and participated avidly in short-track speed skating. On physical examination, there was a palpable, immobile, non-compressible subcutaneous lump. There was no palpable neck, axillary or inguinal lymph node. There was no palpable liver or spleen.
Question 1
What is the next appropriate management step and why?
Answer 1
The imaging modalities and blood tests during the workup process:
- Urgent imaging modality
- Ultrasound of the soft tissue
- Magnetic resonance imaging (MRI) of the abdominal wall
- Computed tomography (CT) of the abdomen with intravenous contrast
- Baseline blood tests
- Full blood count, kidney function test, liver function test
- Tumour markers will be completed if there is a high clinical suspicion of cancer or if histology of the tissue biopsy shows cancer cells
- Tissue biopsy
- Ultrasound-guided fine-needle aspiration biopsy
- Excisional core biopsy
Once results for the above tests have been obtained, and depending on the biopsy results, urgent involvement of the multidisciplinary team (MDT) may be required.
Case continued
The ultrasound revealed a non-compressible and complex cystic lesion in the subcutaneous fat of the right anterior abdominal wall with increased vascularity. MRI of the abdominal wall showed an oval-shaped lesion with a mildly lobulated margin located in the subcutaneous layer, anterior to the lateral margin of the right rectus abdominis muscle, measuring 39 mm × 22 mm × 34 mm (Figure 1). There was a 14-mm increase in size within 10 days between the completion of the ultrasound and the MRI.
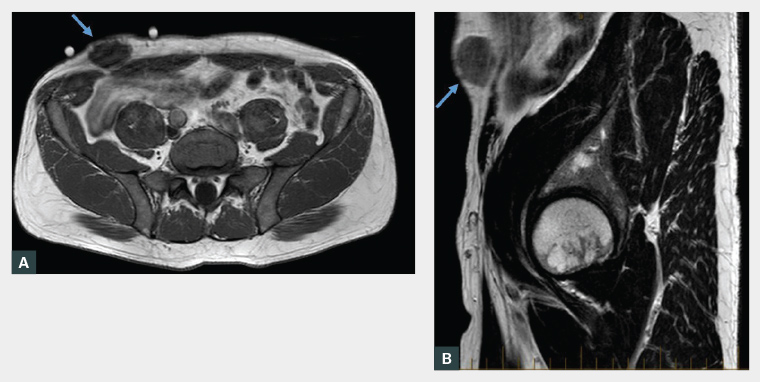
Figure 1. A. Axial and B. sagittal plane magnetic resonance imaging of the abdomen showing the lesion has low signal isointensity relative to muscle and is solitary (arrows).
After discussion, a biopsy was decided against due to the speed of growth and the risk of seeding. The patient subsequently underwent a surgical wide local excision with advancement flap and closure. The lesion was located in the right lower quadrant of the anterior abdominal wall, in the subcutaneous tissue anterolateral to the right rectus abdominis muscle, with no involvement of the underlying muscle. Histopathology showed atypical lymphoproliferative disorder in the dermis and subcutis comprising large atypical cells with frequent mitosis, with no definite involvement of the epidermis; the margins were clear. Flow cytometry was suggestive of B-cell lymphoma. The histological features and immunophenotype were consistent with diffuse large B-cell lymphoma (DLBCL) with an activated B-cell (ABC) phenotype. Detailed immunochemistry and fluorescence in situ hybridisation results were as follows: C-MYC negative; positive for antibodies to CD45, CD20, B cell leukaemia/lymphoma 2 and multiple myeloma 1; and proliferative indicator Ki67 >90%. Epstein–Barr Virus (EBV) and hepatitis B and C virus serology were all negative. The postoperative phase was uneventful. Follow-up CT and positron emission tomography (PET) were completed three weeks after the initial surgery, and showed some expected postoperative changes to the abdominal wall (Figure 2), as well as two areas of abnormal hypermetabolism in the left lung parenchyma with no other nodal or extranodal involvement.
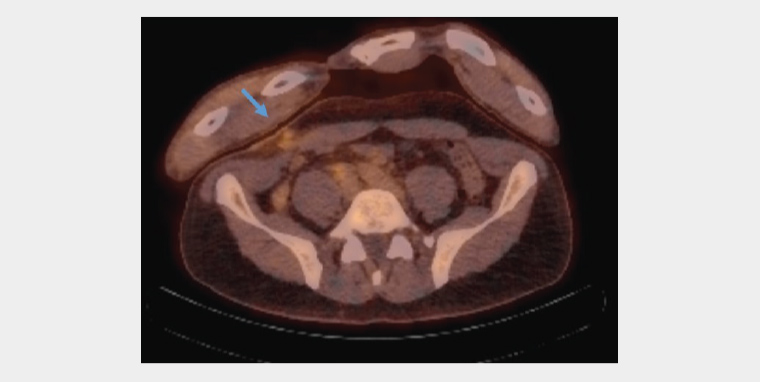
Figure 2. Staging positron emission tomography showed some expected postoperative changes (arrow) on the anterior abdominal wall.
The patient was then discussed in the lymphoma MDT meeting, in which haematologists, radio-oncologists, anatomical pathologists, radiologists and allied health staff were present. Due to the suspicious lesion in the lung, DLBCL-ABC phenotype, high proliferative indicator Ki67 and concerns of remaining DLBCL, chemotherapy (rituximab with cyclophosphamide, doxorubicin, vincristine and prednisone [R-CHOP] 14) was commenced, with granulocyte colony stimulating factor (G-CSF)/filgrastim provided.
The patient was followed up every two weeks for the first month after starting chemotherapy and then monthly until the completion of R-CHOP 14. A six-month post-chemotherapy follow-up revealed the patient had responded well to R-CHOP 14, with no active lesion based on the completed CT and PET scans. The patient is back to working full-time and has started training for his next competition. Evaluation of treatment and surveillance for relapse have been monitored closely by the haematologist with follow-up imaging (eg PET/CT scan) six months after remission in the first year and routine blood tests, including lactate dehydrogenase (LDH), kidney and liver function tests (LFT).
During the treatment and surveillance process, the GP and rheumatologist have been clearly communicating and both have been involved in the monitoring for any signs and symptoms of DLBCL and RA. A nuclear medicine multigated acquisition scan of the heart had been completed before the patient started chemotherapy. That scan showed normal resting biventricular systolic function with an ejection fraction of 53%.
It is critical to monitor for any cardiac toxicity and deterioration of cardiac function during chemotherapy, as well as during methotrexate treatment, in this patient. Other monitoring parameters include LDH, LFT and renal function. The patient’s LDH was mostly normal, except once when it peaked at 280 U/L (normal: 120–250 U/L). Evaluation of renal function at baseline revealed creatinine 90–130 µmol/L (normal: 60–110 µmol/L) and an estimated glomerular filtration rate of 50–75 mL/min/1.73 m2. LFT has been normal throughout.
Question 2
What are the common types of B-cell lymphoma?
Answer 2
- DLBCL (30%)
- Follicular lymphoma (20%)
- Chronic lymphocytic leukaemia (30%)
- Mantle cell lymphoma (5–10%)
- Burkitt lymphoma (1–2% of adult lymphomas; up to 30% of childhood lymphomas)
Question 3
What is the usual first-line treatment for DLBCL and what are other treatment options?
Answer 3
Once DLBCL has been confirmed, R-CHOP 14 may be commenced after consultation with the haematologist.1 The ‘14’ represents treatment being repeated every 14 days per cycle.
Other treatments include bone marrow transplantation or chimeric antigen receptor (CAR) T-cell therapy2 and localised radiotherapy. CAR T-cell therapy, which involves the injection of re-engineered T-cells are back into a patient’s blood, is often reserved for patients who have major side effects from chemotherapy and have failed to achieve remission after two different chemotherapy regimens for DLBCL.
Discussion
Non-Hodgkin lymphoma (NHL) makes up approximately 90% of lymphoma cases in Australia and, in 2022, was the sixth most commonly diagnosed cancer in Australia.3 NHL can be divided into B-cell lymphoma, T-cell lymphoma, indolent lymphoma and aggressive lymphoma. B-cell lymphoma accounts for approximately 85% of all lymphomas, whereas T-cell lymphoma accounts for 15%.4
DLBCL is defined as an aggressive, fast-growing group of large, malignant lymphoid B-cells. DLBCL accounts for 30% of all NHL cases and affects around 2000 Australians every year.2 Other examples of aggressive NHL are mantle cell lymphoma, anaplastic large cell lymphoma and peripheral T-cell lymphomas.4 DLBCL can be divided into different subtypes based on cell surface proteins and genetic mutations. The two main subtypes of DLBCL are ABC (50% of all DLBCL) and germinal cell B-cell (GCB; 30% of all DLBCL).2 Identifying the subtypes and gene mutations of DLBCL are crucial for treatment and prognostication.
According to Lymphoma Australia, DLBCL is the most common type of NHL, but skin or cutaneous B-cell lymphoma is very rare (<1%).2 Based on our literature review, there are no case reports on primary abdominal wall lymphoma involving subcutaneous tissue only, with most case reports being of lymphoma found in the mediastinum, skin or peripheral limbs (predominantly legs).5 The pathophysiology of the development of primary DLBCL is poorly understood. It was hypothesised that DLBCL began as a reactive, inflammatory lymphoproliferative process that subsequently caused lymphogenesis and pseudolymphomas (reactive lymphoid hyperplasias), which may become real lymphomas.5 There are studies describing cutaneous B-cell lymphomas diagnosed in patients treated with methotrexate, in particular for RA.6–9 In many of these patients, EBV has been detected and the regression of lesions has been reported after cessation of methotrexate. This suggests that methotrexate may have induced immunosuppression and subsequently activated lymphoproliferation, which may lead to lymphoma.6–9
Question 4
Is the diagnosis of DLBCL in this patient related to methotrexate?
Answer 4
The patient has a background of RA and has been on methotrexate for 10 years. He does not have EBV or cutaneous DLBCL, although previous studies have suggested that patients using methotrexate for a prolonged period of time have an increased risk of lymphoproliferative disorders, found in the skin secondary to an immunocompromised state.10 From the history reported by the patient, he has not experienced any immunosuppressive-related diseases or hospital admissions prior. His RA has been well controlled and he has been able to compete in high-level sport. Therefore, it is difficult to draw conclusions regarding the development of his abdominal wall DLBCL and his use of methotrexate.
Abdominal wall DLBCL has never been reported in the English literature, but this patient has responded exceptionally well to the treatments provided. His DLBCL is in remission and the 6- to 12-month follow up after the completion of R-CHOP 14 did not show any active lesion on the PET/CT scan. This demonstrates that surgical excision and chemoimmunotherapy have worked effectively in this patient and may be the treatment for abdominal wall DLBCL. Localised radiotherapy could be used to supplement the treatment if required.
Key points
- Ultrasound of the soft tissue and MRI of the abdomen are critical in identifying the abdominal wall mass.
- Urgent involvement of the cancer MDT is imperative in the diagnosis and work-up, treatment planning and care of patients with cancer.
- Primary abdominal wall or cutaneous DLBCL could be treated effectively with surgery and chemoimmunotherapy.11