Case
A boy, aged 10 years, presented with a one-year history of intermittent nocturnal, non-bilious vomiting. The episodes occurred two to three times a month with no obvious triggers or patterns. He would wake at midnight, appear pale, vomit and then return to sleep. There was no associated abdominal pain, headaches, haematemesis, heartburn, dysphagia or urinary symptoms, and no recent travel history. Previous comorbidities included asthma, severe eczema and hay fever in early childhood, which had now resolved. He was on no regular medications. The patient was enjoying Year 5 at school, lived at home with both his mother and father, and had no relevant family medical history. Physical examination was unremarkable with normal vital signs and a soft, non-tender abdomen with no organomegaly. Weight and height were on the 50th percentile, with a consistent trajectory on growth charts.
Question 1
What are the differential diagnoses?
Question 2
What first-line investigations might be indicated?
Answer 1
The unusual symptoms of intermittent recurrent nocturnal vomiting raised suspicion of metabolic disorders and seizures. Some of the differential diagnoses and clinical features that might help narrow down the diagnosis are listed in Table 1.1 Each differential has been classified as common (prevalence ≥1%), uncommon (0.1–1%), rare (0.01–0.1%) or very rare (≤0.01%).
| Table 1. Approach to recurrent vomiting in this patient1 |
| System |
Differential diagnosis |
Features |
| Gastrointestinal |
Eosinophilic oesophagitis |
- Uncommon (prevalence 0.1%)2
- Dysphagia
- Atopy
|
| Inflammatory bowel disease (Crohn disease, ulcerative colitis) |
- Uncommon (prevalence 0.1–0.2%)3
- Crohn disease4
- Abdominal pain
- Diarrhoea +/– blood
- Weight loss/growth failure
- Anaemia
- Extra-intestinal manifestations (eg oral lesions, erythema nodosum, uveitis)
- Ulcerative colitis4
- Colicky abdominal pain
- Bloody diarrhoea
|
| Coeliac disease |
- Common (prevalence 1%)5
- Reduced appetite
- Fatigue/irritability
- Poor growth
- Iron deficiency anaemia
|
| Intestinal malrotation/volvulus |
- Symptomatic malrotation is rare (incidence of intestinal malrotation 0.02%)6
- Bilious vomiting
- Epigastric distension
- Abdominal pain
- Early satiety
- Non-specific in older children (eg recurrent abdominal pain and vomiting)6
|
| Helicobacter pylori-associated peptic ulcer disease |
- Common in certain populations (76% prevalence of H. pylori in Aboriginal and Torres Strait Islander populations)7
- High-risk populations in Australia are migrants from developing countries and Aboriginal and Torres Strait Islander populations7
- Epigastric pain
- Iron deficiency
|
| Post-viral gastroparesis |
- Uncommon (unknown prevalence in children)8
- Recent infection
- Early satiety
- Postprandial epigastric pain
|
| Superior mesenteric artery syndrome |
- Rare (prevalence 0.013–0.3%; true prevalence unknown)9
- Sudden weight loss
- Recurrent postprandial vomiting and abdominal pain
|
| Metabolic and endocrine |
Inborn error of metabolism |
- Rare (prevalence 0.05%)10
- Vomiting induced by prolonged fasting or protein loading
- Neurological symptoms
|
| Adrenal insufficiency |
- Rare (prevalence 0.01–0.02%)11
- Hypoglycaemia
- Hyperpigmentation
- Salt craving
- Nausea
|
| Hereditary fructose intolerance |
- Very rare (prevalence 0.002–0.005%)12
- Vomiting after eating fruits or lollies
|
| Type 1 diabetes |
- Uncommon (prevalence 0.2%)13
- Weight loss, polyuria, polydipsia, dehydration, lethargy
|
| Neuropsychiatric |
Cyclical vomiting syndrome |
- Common (prevalence 1.9–2.3%)14
- Stereotypical vomiting episodes, with each episode appearing similar to previous in terms of the onset, frequency and duration of vomiting
- Family history of migraines
|
| Space-occupying lesion |
- Very rare (prevalence 0.005%)15
- Early morning vomiting
- Headache
- Visual disturbances
- Nystagmus
|
| Early-onset benign childhood occipital epilepsy (Panayiotopoulos syndrome) |
- Rare (prevalence 0.02–0.03%)16
- Triad of nocturnal seizures, tonic eye deviation, vomiting
|
| Each differential has been classified as common (≥1%), uncommon (0.1–1%), rare (0.01–0.1%) or very rare (≤0.01%). The percentage (%) refers to the general prevalence of the disease in children and adolescents. |
Answer 2
Indicated first-line investigations include:
- finger prick blood glucose level4
- bloods: full blood count, electrolytes, fasting glucose, liver function tests, C-reactive protein, amylase, lipase, iron studies, coeliac serology
- urine and stool microscopy, culture and sensitivity (MCS)
- abdominal ultrasound4 (if relevant to differential diagnosis).
If a space-occupying lesion is suspected, consider urgent magnetic resonance imaging (MRI) of the brain and urgent paediatric referral.4
Case continued
Blood investigations were essentially normal besides mild eosinophilia. Urine MCS and abdominal ultrasound were normal. Due to the unusual presentation of nocturnal vomiting, a brain MRI was conducted to exclude a space-occupying lesion. The brain MRI was normal in this patient.
The patient was subsequently referred to gastroenterology, where an upper gastrointestinal endoscopy revealed linear furrows in the oesophagus, with the biopsy confirming eosinophilic oesophagitis (EoE; Figure 1).
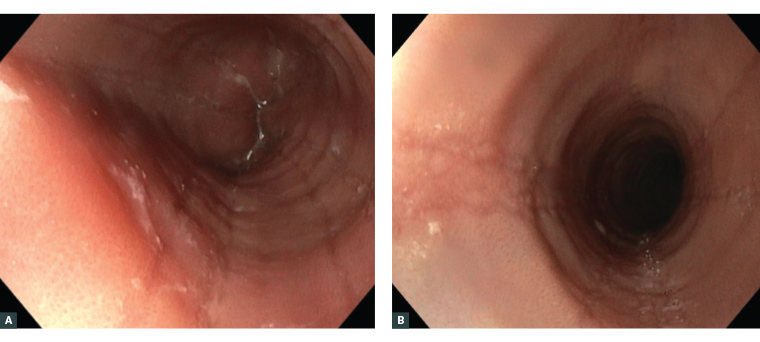
Figure 1. Common endoscopic findings in eosinophilic oesophagitis.17
A. White plaques/exudates and oedema/decreased vascular markings. B. Linear furrows and oesophageal rings (trachealisation) caused by thickening of the mucosa and submucosa.
Question 3
What is EoE?
Question 4
What symptoms occur in EoE?
Question 5
How is EoE diagnosed?
Question 6
How is EoE managed?
Question 7
What are the implications and complications for the patient?
Answer 3
EoE is a chronic T helper 2 cell immune-mediated disease caused by increased eosinophils in the oesophageal epithelium.18 Exposure to certain foods or airborne allergens stimulates a cytokine cascade that disrupts the epithelial barrier, causing eosinophilic inflammation in the oesophageal mucosa and submucosa.18,19 EoE is increasing in prevalence, occurring in 1 in 1000 Australian children.2
Answer 4
The symptoms of EoE can vary according to the patient’s age. Symptoms have also been shown to poorly correlate with endoscopic and histological findings because EoE involves a delayed hypersensitivity reaction.20 Symptoms of EoE in children are presented in Table 2.
| Table 2. Symptoms of eosinophilic esophagitis in children21 |
- Food refusal
- Prolonged mastication
- Dysphagia
- Food bolus impaction
- Vomiting/regurgitation
- Abdominal pain
- Poor weight gain
|
Answer 5
The diagnosis of EoE is made if:
- symptoms of oesophageal dysfunction are present
- there are ≥15 eosinophils per high-power field on biopsy
- other causes of oesophageal eosinophilia are excluded.22
Answer 6
The aim is to manage symptoms and prevent oesophageal fibrosis. Treatment is patient dependent and long-term maintenance therapy is often necessary.23–26
Proton pump inhibitors
Proton pump inhibitors (PPIs; eg omeprazole, esomeprazole) have anti-inflammatory effects in conjunction with their acid suppression role. PPIs alone are moderately effective in EoE, with up to 50% of patients achieving clinical and histological remission.23 An 8- to 12-week trial of PPI therapy is usually considered first-line therapy. The optimal dosage and duration of therapy with PPIs is yet to be validated.24 Omeprazole, esomeprazole or lansoprazole doses of 10 mg twice daily for children weighing 10–20 kg and 20 mg twice daily for children weighing >20 kg have been recommended.25
Food elimination diet
Dietician review is necessary for supervision of a food elimination diet, as well as ensuring all other nutritional requirements are met. Common food triggers are cow’s milk, wheat, egg and legumes. Skin prick testing has a limited role in informing EoE triggers.24 Options for elimination diets include two, four or six foods.
The two-food elimination diet eliminates milk and gluten. Being less restrictive, it can be trialled before stepping up to the four- or six-food elimination diets. The four-food diet excludes milk, egg, soy and wheat. The six-food diet excludes nuts and fish/shellfish as well, with 74% of patients achieving clinical and histological remission.24
Repeat endoscopy is required at six to eight weeks to determine histological remission. If in remission, a food group is reintroduced, followed by a repeat endoscopy.24
Swallowed glucocorticoids
Swallowed glucocorticoids (eg fluticasone, budesonide) are second-line agents if EoE is refractory to PPIs. Swallowed glucocorticoids act by coating the oesophagus to reduce symptoms and inflammation. Eating and drinking should be avoided 30–60 min after administration to avoid washing away the medication.26 Fluticasone metered dose inhalers can be sprayed in the mouth and swallowed. Doses of 50 µg twice daily for children aged one to four years, 125 µg twice daily for those aged 5–10 years and 250 µg twice daily for those >10 years of age are recommended.25 Budesonide is given at a dose of 0.5 mg twice daily in children, with half the dose given after two to three months of initiation for maintenance treatment.25 Pastes and slurries, such as Splenda (sucralose sweetener; Heartland Food Products Group, Fort Washington, MD, USA) can be used to aid the swallowing of corticosteroids.25 Steroid efficacy ranges from 53% to 95% after 2–12 weeks of treatment.22 Additional benefits include a reduction of tissue remodelling and an improvement in the oesophageal mucosal barrier. Steroids are associated with oesophageal candidiasis in 15% of patients.27
If EoE is refractory to treatment, or if oesophageal stenosis is present, oesophageal balloon dilatation is considered.21,24 The widening of the oesophageal diameter can provide long-lasting relief of symptoms, but does not provide histological improvement.28
In addition, there are emerging therapies involving monoclonal antibodies to target specific cytokines.27
Answer 7
Complications of EoE include:
- food impaction
- oesophageal stricture, perforation or fibrosis
- dysfunctional feeding and malnutrition (especially in children)
- steroid adverse effects.22
Case continued
The patient was treated with omeprazole and the six-food elimination diet, achieving histological remission at eight weeks. Dairy reintroduction caused the recurrence of EoE. The evaluation revealed that dairy was the trigger food and, once eliminated, the patient was asymptomatic and had normal oesophageal histology. All other food allergens were successfully reintroduced without endoscopic evidence of recurrence. He remained in clinical remission on omeprazole and a dairy-free diet.
Key points
- EoE should be considered in atopic children presenting with non-specific gastrointestinal symptoms including unexplained vomiting.18
- Diagnosis is established through upper gastrointestinal endoscopy and oesophageal histology.
- EoE is a chronic condition requiring long-term therapy with PPIs, a food elimination diet and/or topical glucocorticoids.21