Diabetic peripheral neuropathy (DPN) affects 31% of people with type 2 diabetes (T2D).1 DPN contributes to reduced quality of life and higher annual healthcare costs,2 and is an independent predictor of all-cause and diabetes-related mortality.3 Promotion of physical activity in adults with diabetes is an important public health strategy because the amount and intensity of physical activity is inversely associated with the risk of all-cause and cardiovascular disease mortality.4 Unfortunately, a large majority (73%) of people with DPN report difficulties with physical function (ie walking, exercise, standing and balance).5 Wearable technology is now available to provide personalised information about whether an individual is achieving health-enhancing levels of physical activity. Personal Activity Intelligence (PAI; www.ntnu.edu/cerg/personal-activity-intelligence) is a heart rate-based technology that converts an individual’s heart rate responses to physical activity into a personalised and easily understandable metric, known as PAI.6 Data from 3133 patients with cardiovascular disease identified that participants with weekly PAI scores of ≥100 had a 24% lower risk of all-cause mortality.7 In low-active adults with T2D, PAI self-monitoring combined with four two-hour sessions involving exercise tasters and behaviour change counselling resulted in increased exercise capacity compared with a control group.8 Although this study supports the use of eHealth approaches, patients with DPN are often excluded or more likely to drop out of exercise studies.9
This study aimed to investigate the feasibility, acceptability, safety and impact on foot symptoms of an eHealth intervention involving PAI, exercise tasters, behaviour change counselling and PAI self-monitoring in people with DPN. It was hypothesised that participants would be satisfied with the eHealth program and PAI technology and be able to achieve health-enhancing levels of physical activity without major adverse events or worsening of foot symptoms.
Methods
This prospective 12-week cohort study was approved by the human research ethics committees at Griffith University (2019/214) and The University of Queensland (2019001070) and conducted in accordance with the Declaration of Helsinki. The protocol, including COVID-19 impact and amendment from a randomised controlled trial to a single-arm study, was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12619001300167).
Participants located in Brisbane, Australia, were recruited between September 2019 and January 2020 using university and local diabetes agency newsletters and social media. To be eligible for inclusion, participants had to be community-dwelling adults aged 30–80 years, diagnosed with T2D by their doctor, not meeting physical activity guidelines (210 minutes per week of moderate-intensity exercise or 125 minutes per week of vigorous intensity exercise)10 and with definite or probable diabetic neuropathy, based on sensory or nerve conduction tests (described below). Participants with current leg ulcers or any condition preventing physical activity performance as outlined elsewhere8 or unable to complete study requirements were excluded. Participants attended two screening sessions and written medical clearance was sought from their general practitioner or specialist.
At the first session, HbA1c was measured from a finger prick blood sample using a point-of-care device (Afinion 2 Analyzer; Abbott, Oslo, Norway), followed by assessment of blood pressure, anthropometry and physical tests. Next, quantitative sensory tests and nerve conduction tests were performed according to published recommendations (Sierra Summit, Cadwell, WA, USA).11,12 Criteria for DPN were any of the following: absence of the Achilles tendon reflex; absence of 5.07 (10-g) monofilament sensation (Aesthesio; Danmic Global, San Jose, CA, USA) or vibration perception lower than 4 using a 64-Hz tuning fork (Rydel-Sciffer tuning fork; US Neurologicals, Poulsbo, WA, USA) over the plantar aspect of one or both third metacarpals; or abnormality of any attribute of nerve conduction in two separate nerves, one of which had to be the sural nerve.12 At the second session, a cardiopulmonary exercise test (CPET) was conducted to screen for coronary artery disease, assess cardiorespiratory fitness (Vo2 peak) and determine peak heart rate (used in the PAI algorithm). Heart rate recovery (decrease from peak) at two minutes after exercise was recorded to assess autonomic nervous system function. A detailed description of the CPET, which was supervised by a medical doctor, is outlined elsewhere.8
Intervention
The intervention closely resembled the PAI eHealth program evaluated in a previous trial with people with T2D.8 Participants were invited to attend four, in-person, two-hour weekly group sessions jointly facilitated by a musculoskeletal physiotherapist and an accredited exercise physiologist at the university. At their first session, participants were given a fitness wristband that measures heart rate (Lynk2; Accuro, Oakbrook Terrace, IL, USA) and downloaded a research version of the PAI app (PAI Research; PAI Health, Vancouver, Canada) on their smartphone. Each session during the intervention period (Weeks 1–4) consisted of three core activities summarised in Table 1: PAI learning, PAI playtime and PAI behaviour change counselling. PAI learning involved education, discussion and troubleshooting of the self-monitoring. Participants were informed that maintaining a PAI score ≥100 has the strongest health benefits, but progressive benefits could also be achieved with maintaining lower levels (25, 50 or 75 PAI). During PAI playtime, participants were guided through different types and intensities of exercise, demonstrating that the higher the intensity of exercise, the faster the accumulation of PAI points. The PAI behaviour change counselling used cognitive and behaviour change techniques to promote self-managed exercise.13 After completion of the four sessions, participants were encouraged to self-direct their physical activities and exercise using PAI monitoring during Weeks 5–12, and could telephone the research team for support if they experienced unresolved technical issues.
| Table 1. Core activities included within each weekly two-hour PAI eHealth session |
| Objective |
Information/activities |
| PAI learning |
| To understand the PAI concept, collect and interpret PAI data to inform decisions regarding self-directed physical activity |
- Information about the PAI algorithm, associations between PAI scores and reduced risk of premature mortality
- Assistance to download the PAI Research app on their smartphone, activate heart rate recording, sync with the PAI Research app and troubleshoot technical problems
- Discussion on interpretation of PAI scores and physical activities completed
|
| PAI playtime |
| To experience different types and intensities of exercise and appreciate the different ways PAI points can be accumulated |
- Supervised exercise (~20 minutes) with assistance to activate heart rate recording and syncing
- Week 1: Low-intensity, continuous aerobic exercise (eg walking)
- Week 2: High-intensity interval training via exercise bike or treadmill
- Week 3: Resistance exercises of large muscle groups with active recovery (eg stairs)
- Week 4: Exercise of choice
- Measurement of blood glucose and blood pressure before and after exercise
- Tailored feedback on exercise technique from a physiotherapist and accredited exercise physiologist
- Provision of resistance bands (TheraBand) for home use
|
| PAI behaviour counselling |
| To enable self-directed physical activity and PAI self-monitoring |
- Group exercise counselling using the 5As framework (Assess, Advise, Agree, Assist, Arrange)13
- Assess and advise: On knowledge and beliefs about exercise; diabetes and diabetes-related complications; exercise recommendations; exercise concerns (eg fear of injury or pain)
- Agree: Collaborative exercise goal setting
- Assist: To identify values and preferences for physical activity to accumulate PAI; reinforcing effort in monitoring and achievement (≥100 PAI or some physical activity is better than none); problem-solve exercise barriers (eg fear of injury or pain, negative emotions); support physical activity maintenance and relapse/coping plans
- Arrange: Telephone support for PAI technical issues
|
| PAI, Physical Activity Intelligence. |
Outcomes
Feasibility
Participant attendance records were kept at group sessions. Data were downloaded from the PAI Research database to compute wearable wear-time, average daily PAI and percentage of days ≥25, 50, 75 or 100 PAI during Weeks 2–4 and 5–12. Because PAI is scaled across a seven-day rolling window, the first seven days of data were excluded. The percentage of daily PAI that was achieved from low-, moderate- and high-intensity physical activity was also extracted for Weeks 2–4 and 5–12. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v5.0)14 using data collected during eHealth sessions (Weeks 1–4) or if participants contacted research staff during Weeks 5–12.
Acceptability
At Week 4, participants completed a written questionnaire to rate their satisfaction with the eHealth intervention (five-point Likert scale, endpoints ‘very dissatisfied’ to ‘very satisfied’) and the PAI technology using the Technology-based Experience of Need Satisfaction – Interface (TENS-Interface) questionnaire, with scores ranging from 5 to 25.15
Impact
Six of the most common symptoms of DPN experienced in the feet (aching pain, burning pain, shooting pain, prickling/tingling, sensitivity to touch and numbness) were evaluated at Weeks 0 and 12. To reduce recall bias when evaluating fluctuating symptoms, ecological momentary assessment was used,16 whereby repeated mobile phone surveys were delivered using a freely available app (https://pielsurvey.org). After receiving a prompt on their smartphone five times per day for seven days, participants were asked to rate the intensity of each symptom using visual analogue scales, with endpoints of 0 (‘none’) and 1 (‘severe’). Mean values over the seven days were computed.
Statistical analysis
Daily PAI scores and the percentage of days meeting thresholds of ≥25, 50, 75 and 100 PAI were summarised as the median and interquartile range (IQR). Daily PAI scores were compared between Weeks 2–4 and Weeks 5–12 using the Wilcoxon matched-pairs signed-rank test. Symptom intensity ratings were summarised as the mean±standard deviation and paired t-tests used to compare data at Weeks 0 and 12 using intention to treat. Effect sizes (Cohen’s d) were calculated (t/sqrt(n)) and interpreted as small (0.2–0.49), medium (0.5–0.79) or large (≥0.8).
Results
The flow of participants from enrolment through to analysis is illustrated in Figure 1. Ten individuals consented and their characteristics are listed in Table 2. Mean attendance at the eHealth sessions was 3.5 of four sessions. One participant did not attend any eHealth sessions due to work commitments and another participant attended two sessions, then withdrew due to increased anxiety over tracking of their heart rate. The remaining participants were ‘very satisfied’(n=7) or ‘satisfied’(n=1) with the eHealth intervention. Participants reported high competence and satisfaction using the PAI technology (TENS-Interface mean 21.0±1.5).
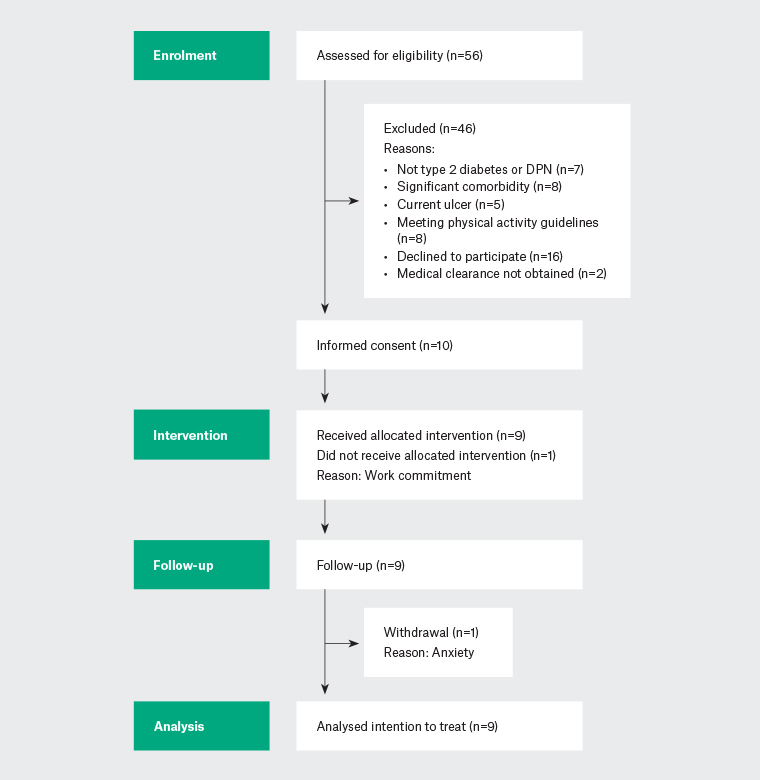
Figure 1. Participant flow diagram.
DPN, diabetic peripheral neuropathy.
| Table 2. Participant characteristics: Frequency (%), mean±SD or median [IQR] |
| Demographics |
| No. men/women |
5/5 |
| Age, years |
61.7±6.3 |
| Body mass (kg) |
100.6±23.5 |
| Body mass index (kg/m2) |
33.7±6.1 |
| Height (cm) |
170.5±12.0 |
| Waist circumference (cm) |
114.6±17.4 |
| Systolic BP (mm Hg) |
138±18.7 |
| Diastolic BP (mmHg) |
81.6±10.6 |
| Diabetes characteristics |
| Duration (years) |
11.1±9.1 |
| HbA1c (%) |
7.1 [6.4–7.7] |
| Cardiopulmonary exercise test |
| Vo2peak (mL/kg/min) |
20.7±6.9 |
| % of APHR peak |
89.9±5.5 |
| Peak heart rate |
154±10.9 |
| Heart rate recovery at 2 min |
40.2±2.8 |
| Comorbidities |
| Coronary artery disease |
2 (20) |
| Arthritis or musculoskeletal conditions |
6 (60) |
| Hypertension |
5 (50) |
| Eye/vision problems |
5 (50) |
| Hypercholesterolemia |
8 (80) |
| Respiratory problems |
3 (30) |
| Anxiety or depression |
4 (40) |
| History of cancer |
2 (20) |
| Medications |
| Insulin |
1 (10) |
| Biguanide |
9 (90) |
| Sulfonylurea |
2 (20) |
| HMG-CoA reductase inhibitor |
9 (90) |
| Serotonin receptor agonist |
2 (20) |
| Beta-blocker |
3 (30) |
| ACE inhibitor |
4 (40) |
| Calcium channel blocker |
2 (20) |
| Selective β2-adrenoceptor blocker |
2 (20) |
Unless indicated otherwise, data are presented as n (%), mean±SD or median [IQR].
ACE, angiotensin-converting enzyme; APHR, age-predicted heart rate; BP, blood pressure; HbA1c, glycated haemoglobin; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A; IQR, interquartile range; SD, standard deviation. |
Median (IQR) wearable wear-time was 100% (95–100%) and 97% (82–100%) in Weeks 2–4 and 5–12, respectively. Median (IQR) PAI scores of 92.7 (80.2–144.9) and 70.7 (24.3–132.5) were achieved in Weeks 2–4 and 5–12, respectively, with no significant difference (P=0.16). The percentage of days meeting thresholds of ≥25, 50, 75 or 100 PAI is illustrated in Figure 2. In Weeks 2–4, daily PAI was achieved from a combination of high- (43%) and moderate-intensity (48%) physical activity, with only 9% from low-intensity physical activity. In Weeks 5–12, daily PAI was achieved from high- (38%), moderate- (51%) and low-intensity (11%) physical activity.
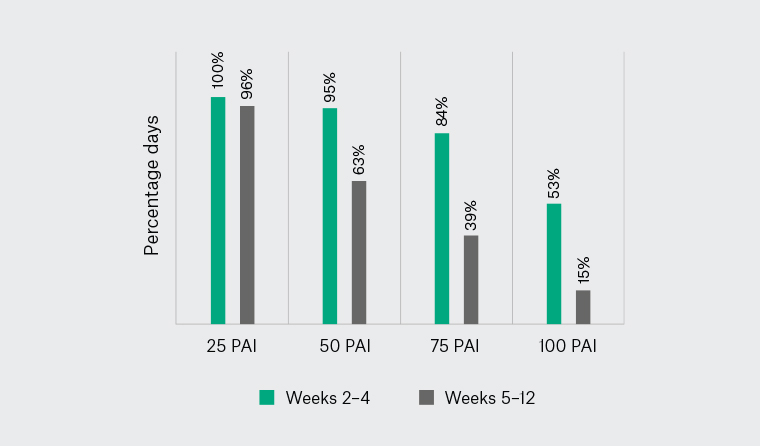
Figure 2. Median percentage of days meeting Physical Activity Intelligence (PAI) scores of 25, 50, 75 or 100 during Weeks 2–4 (in-person intervention period) and Weeks 5–12 (self-directed period).
No Grade 3 or higher adverse events occurred. Grade 2 adverse events included tachycardia after CPET requiring non-urgent medical intervention (n=1) and knee effusion from a fall requiring non-invasive intervention (n=1). Twelve Grade 1 events included: hypoglycaemia after exercise, resolving with fast acting carbohydrate (n=5); symptomatic hypotension before exercise, resolving upon commencing exercise (n=1); and musculoskeletal pain not requiring intervention (n=6).
Mean intensity ratings of foot symptoms are presented in Table 3. Large reductions in deep aching (d=–1.11, P=0.02) and burning pain (d=–0.93, P=0.03) were observed. Reductions were also observed in shooting pain (d=–0.71) and sensitivity to touch (d=–0.69), but these did not reach statistical significance (P<0.09). There were no changes in prickling/tingling or numbness in the feet.
| Table 3. Self-reported intensity of the most common symptoms of diabetic peripheral neuropathy in the feet at Weeks 0 and 12 |
| Symptom intensity |
Week 0 |
Week 12 |
P-value |
Cohen’s d |
| Deep aching pain |
0.24±0.19 |
0.13±0.18 |
0.02 |
–1.11 |
| Sensitivity to touch |
0.17±0.14 |
0.11±0.16 |
0.09 |
–0.69 |
| Burning pain |
0.26±0.25 |
0.16±0.23 |
0.03 |
–0.93 |
| Shooting pain |
0.21±0.18 |
0.11±0.15 |
0.08 |
–0.71 |
| Prickling/tingling |
0.22±0.21 |
0.15±0.18 |
0.12 |
–0.60 |
| Numbness |
0.22±0.22 |
0.24±0.21 |
0.82 |
0.08 |
| Unless indicated otherwise, data represent mean±standard deviation intensity measured on a visual analogue scale (0–1) collected via repeated mobile phone surveys over seven-day periods. |
Discussion
High attendance at in-person sessions, high overall satisfaction and competence using PAI technology and high wearable wear-times indicate the PAI eHealth intervention is feasible in people with DPN. Although major adverse events were not observed, minor adverse events, including hypoglycaemia and musculoskeletal problems, were common, indicating that supervision is important when low-active individuals with DPN commence an exercise routine.
During the intervention period (Weeks 2–4), participants achieved ≥50 PAI on 95% of days and ≥100 PAI on 53% of days. These rates are considered clinically meaningful because on enrolment participants were not meeting physical activity guidelines, and PAI scores ≥50 are associated with beneficial health effects.6 Indeed, a positive association between PAI and cardiorespiratory fitness (Vo2peak) in both men and women suggest that PAI is a useful tool for quantifying the amount of physical activity needed to produce significant health benefits.17 During Weeks 5–12, participants achieved ≥50 PAI on 65% of days but ≥100 PAI on only 15% of days. It must be acknowledged that this period coincided with the introduction of social distancing requirements due to COVID-19 across Australia. A reduction in physical activity during the first months of social distancing has been previously shown to be more pronounced among overweight/obese individuals with chronic disease.18 Nevertheless, the decline in PAI scores indicates more work is needed to sustain self-directed physical activity in people with DPN.
Heart rate responses to exercise might be influenced by comorbidities such as cardiac autonomic neuropathy or beta-blocker medications. Data from the CPET indicated that participants with DPN reached a mean of 90% of their age-predicted heart rate maximum,19 which is an appropriate heart rate response for low-active individuals with significant comorbidities. This suggests that using an equation to predict maximum heart rate would provide enough accuracy to use the PAI approach when data from a maximal test (CPET) is not available. One participant had an impaired heart rate recovery suggesting possible cardiac autonomic neuropathy. However, this individual had an appropriate peak heart rate (94% of age-predicted value) and achieved an average of 77.5 PAI over the 12 weeks, indicating that the heart rate monitoring approach was still valid.
Study limitations included the small sample size and lack of a control group; therefore, we cannot be certain that the improvements in symptoms were the direct result of the intervention. Because this was a feasibility study, formal sample size calculations were not conducted. Effect sizes are provided to inform a future trial with a usual-care control group. Point estimates suggest the intervention might lead to moderate to large changes in positive but not negative symptoms of DPN. Limitations of the PAI technology included connectivity issues and an inability for participants to view information outside the last seven days on the app, which might have affected tracking of long-term physical activity behaviours. Finally, the behaviour change counselling was delivered by health professionals not traditionally trained in this discipline, which might have constrained impact.
PAI provides user-friendly, quantifiable feedback on an individual’s daily physical activity, which might serve as a motivator to reduce their long-term health risk. PAI might also serve as an important tool in health management in primary care. Sharing this metric with general practitioners might lead to a discussion of physical activity goals, barriers and enablers and tracking of progress over time. General practitioners are also encouraged to enable patients to connect with other allied health professionals (eg accredited exercise physiologists, physiotherapists and health psychologists) who can collaborate to promote physical activity. Further studies are needed to implement and test whether use of the PAI eHealth program in primary care can enhance public health.
Conclusion
An innovative, brief eHealth program integrating PAI, exercise tasters, behaviour change counselling and self-monitoring of heart-rate responses to exercise was acceptable and feasible in low-active adults with DPN. Participants achieved health-enhancing levels of physical activity, reported reduced intensity of painful foot symptoms and had no serious adverse events. However, a decline in PAI scores after completion of the four-week in-person program indicates additional strategies are needed to maintain self-directed physical activity behaviour.