Globally, liver cancer is the seventh most frequently occurring cancer and the second most common cause of cancer mortality.1 Incidence and mortality rates for liver cancer have increased rapidly in Australia;2 since the mid-1980s, the age-standardised incidence rate has increased by more than 300% and the mortality rate has increased by almost 200%.3 Hepatocellular carcinoma (HCC) is the most common form of liver cancer, accounting for 85% of cases.4 Both globally and in Australia, the five-year survival rate for HCC is 20%, the fifth lowest survival rate of all cancers.5,6
The low HCC survival rate is largely due to delayed diagnosis, because people remain asymptomatic during the early stages of HCC and clinical examination and investigations might not detect any abnormalities.7–9 This often delays the detection of the tumour(s) until advanced stages.10 To mediate this, HCC surveillance is recommended in high-risk populations, such as patients with liver cirrhosis and/or chronic hepatitis B (CHB; Box 1). HCC surveillance consists of liver ultrasound scans (USS), preferably with the serum biomarker α-fetoprotein (AFP), performed every six months.11–15 Despite recommendations, HCC surveillance rates have remained low. A meta-analysis on the utilisation of HCC surveillance for cirrhotic patients in the US reported a pooled rate of just 18.4%.16 However, utilisation rates were sixfold higher when provided within a surveillance program (eg reminder systems) compared to usual care.17
| Box 1. Target populations for hepatocellular carcinoma surveillance11 |
- People with cirrhosis (any aetiology)
- People living with chronic hepatitis B without cirrhosis in:
- Aboriginal and Torres Strait Islander people aged >50 years
- Asian men aged >40 years
- Asian women aged >50 years
- People born in sub-Saharan Africa aged >20 years
|
Primary care providers (PCP) play an important role in disease prevention and management, and are generally the first point of contact patients have with the health system.18 In the context of HCC surveillance, PCPs have a crucial role in diagnosing and managing viral hepatitis infections and cirrhosis, two of the key risks for HCC. Therefore, PCPs might also play an important role in conducting surveillance for HCC.
Many studies have evaluated the effectiveness of HCC surveillance in the primary care setting. Several of these19–25 have attributed underutilisation of surveillance to both patient (eg poor adherence, cost) and PCP (lack of awareness of surveillance guidelines) factors. Therefore, the aim of this review was to synthesise the evidence for HCC surveillance in primary care settings and identify barriers and facilitators to HCC surveillance from the perspectives of PCPs and patients.
Methods
The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement,26 and the study protocol was registered with PROSPERO (CRD42020204195). Database searches were conducted from inception to August 2020. In October 2022, the search was updated to include publications up to that date. Five biomedical databases, the Centre for Reviews and Dissemination website and the grey literature were searched; full details are provided in Appendix 1). Interventional, observational and qualitative studies reporting on HCC surveillance undertaken within primary care settings were included in this review.
Publications were eligible for inclusion in the review if they reported on any HCC surveillance activity (USS, Fibroscan, magnetic resonance imaging [MRI], computed tomography [CT], other imaging interventions, AFP, zinc sulfate turbidity test, behavioural interventions to increase participation) that occurred in a primary care setting. In turn, PCPs included general practitioners and internal medicine and family medicine practitioners. Depending on the health system, primary care was included when provided in community and tertiary settings. Review articles, articles reporting on hospital-based screening, screening in specialist clinics and outpatient clinics and those focusing on paediatric populations were excluded from the study.
Titles and abstracts were independently reviewed against the eligibility criteria by two reviewers (PD, KM). Discrepancies were resolved by a third reviewer (BdG). A full text review was then conducted for identified papers by three reviewers (PD, BdG, KM), with any discrepancies resolved by KM. The PRISMA flow diagram in Figure 1 provides an overview of this process. Data were extracted using the Covidence extraction tool by two independent reviewers (MM, JW) and collated by MM and PD.
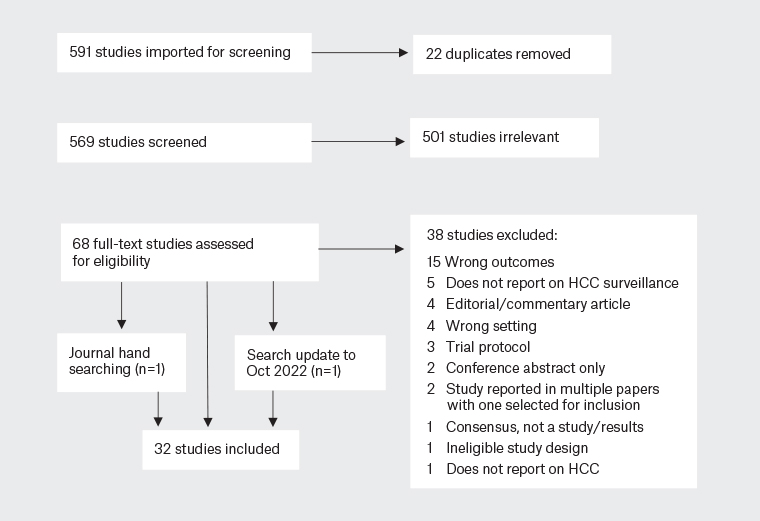
Figure 1. PRISMA flow diagram of study selection.
HCC, hepatocellular carcinoma.
Study quality was assessed by two independent reviewers (NK, MM) using the National Institute for Heath and Care Excellence (NICE) quality appraisal checklists.27 Assessment followed a structured approach and emphasised evaluation of potential biases that were most likely to affect results. A narrative synthesis was undertaken due to the heterogeneity in the eligible studies.
Results
Initial searches yielded a total of 591 records. Following title and abstract review, 68 studies were assessed for eligibility. In all, 32 studies were included in this review (Figure 1).19–25,28–52
General characteristics of the included studies
Summaries of the included papers are provided in Appendix 2 (outcomes) and Appendix 3 (study design). Most studies originated from the USA20–25,28–32,34–36,38–46,48–52 (88%; n=28), with two studies from Australia19,33and single studies from Italy37 and Japan.47 The most common study designs were retrospective cohort (44%; n=14), cross-sectional (28%; n=9) and quasi-experimental (14%; n=4). Study populations were patients (63%; n=20), healthcare providers (HCPs; 31%; n=10) or a combination of both (6%; n=2). For studies focusing on patient groups, 34% (n=11) focused on patients with cirrhosis and 28% (n=9) focused on CHB patients. For HCP studies (n=10), seven focused on PCPs and the remaining studies reported on both PCPs and other HCPs.
Quality appraisals
The internal validity of two studies was assessed as having significant potential sources of bias.44,49 Assessment of the remaining studies indicated that aspects of the study design and conduct were such that the risk of bias was minimised. The risk of bias also flowed through to the external validity of one study,44 precluding the ability to generalise its results beyond the study population.
Patient-focused studies (n=21)
Viral hepatitis patients (n=10)
Studies mainly focused on CHB patients (n=10) and were conducted in the USA20,21,28–32 (n=8) and Australia19,33 (n=2). Studies focused predominantly on culturally and linguistically diverse (CALD) cohorts20,21,29,32 (n=5) and cohorts of Asian ancestry30,31,33 (n=3). Surveillance approaches included USS six-monthly,33 USS 6- to 12-monthly,21 USS ± AFP six- monthly,19 one or more USS and/or AFP annually,20,30 one or more abdominal imaging study ± AFP annually32 and USS ± AFP without frequency specified.29
An Australian study showed a management tool to support shared care of CHB patients in primary care with remote oversight from hospital-based gastroenterologists improved rates of surveillance.33 Surveillance increased from 26% to 88% after the intervention. A second Australian study evaluated a program to support HCC surveillance.19 This included specialist nurses contacting patients lost to follow-up, strengthening reminder systems by mailing radiology/pathology requests and regular reviews and telephone calls to patients not attending.19 Over 4.5 years, 27% of participants were reported to have ‘good’ adherence to surveillance (average of one or more USS per 7 months), 43% had ‘suboptimal’ adherence (1–2 USS every 14 months) and 30% had ‘poor’ adherence (an average of less than one USS per 14 months).19 Of note, half the patients who had regular hepatitis B (HBV) viral load tests had suboptimal or poor adherence to HCC surveillance.19 This was likely related to accessibility: pathology samples were collected within the clinic, whereas USS was conducted in a different setting.
A CHB registry with HCC surveillance workflows accompanied by follow-up with PCPs and patients in a primary care site that served migrant populations was evaluated.34 Comparator groups were patients managed by gastroenterologists (no recent PCP visit) and a PCP usual care group. Before implementation, surveillance uptake was 27% for the intervention group, 22% for the gastroenterologist group and 3% for the PCP usual care group. After implementation, surveillance increased to 34% for the intervention group, decreased for the gastroenterologist group (15%) and remained stable for the usual care group (2%).34 Staff provided feedback that the increased workload of providing follow-up in a busy clinical setting was unsustainable.
A retrospective study evaluated surveillance over a 10-year period.30 The rate in years 1, 2 and 10 was 67%, 47% and 24%, respectively. Patients undergoing surveillance were more likely to be diagnosed at an early stage than those not undergoing surveillance (79% vs 19%), receive curative treatment (71% vs 30%), and have greater median survival (1624 vs 111 days).30 Another study reported 63% of high-risk CHB patients underwent surveillance over 12 months.21 A survey with PCPs was also conducted: PCPs of Asian ethnicity and a positive attitude towards surveillance were positively associated with HCC surveillance.21
Four studies used retrospective cohorts to compare surveillance rates between PCPs and non-PCP specialists, predominantly gastroenterologists and hepatologists.21,29,31,32 Overall, rates were higher when patients were managed by a non-PCP specialist. Data from a Veterans Affairs (VA) healthcare system showed that 22% of HCC cases were diagnosed through surveillance, with patients managed by hepatologists more likely to receive surveillance (75%) than those managed by PCPs (25%).29 In another study, surveillance adherence rates over 18 months were relatively high for both PCPs (73%) and non-PCP specialists (92%).31 Focus groups identified barriers to surveillance including under-recognition/diagnosis of CHB, lack of continuity of care, inadequate trust within patient–doctor relationships and infrequent patient visits.31 A third study showed patients managed by gastroenterologists were more likely to undergo timely surveillance compared with PCP management, with an odds ratio (OR) of 6.87 (95% confidence interval [CI]: 4.5, 9.7).32 Further, 74% of non-adherence was due to PCPs not ordering surveillance and 12% was due to gastroenterologists not ordering this.32
Cirrhotic patients (n=11)
Almost all studies were conducted in the USA (n=10), and participants were predominantly White35,36,38,39,44 or from CALD backgrounds.40,41,43 Surveillance included six-monthly USS,41 six-monthly42,43,45 or more frequent38,39 abdominal imaging (USS, CT or MRI) or abdominal imaging ± AFP within one year of cirrhosis diagnosis44 or annually.35,36
A randomised trial compared surveillance supported by mailed outreach + patient navigation with usual care by both PCPs and gastroenterologists.43 Surveillance participation for the intervention and usual care group was 23% and 7%, respectively.
One study evaluated the impact of a reminder system for PCPs on surveillance participation.38 The intervention increased the surveillance rate from 18% to 28%, compared with an increase from 16% to 18% in the control group. A training program for PCPs that aimed to increase diagnosis of cirrhosis, referral to hepatologists and surveillance for HCC was evaluated.37 Before the intervention, 35% of HCC cases were diagnosed through surveillance; after the intervention this increased to 55%. For controls, pre- and postintervention rates were 26% and 20%, respectively. HCC diagnosed at an early stage increased from 48% to 64% in the intervention group, and from 38% to 43% in the control group.37 The five-year survival rate increased from 20% to 40% in the intervention group and remained unchanged in the control group (20%).37
A retrospective study evaluated the uptake and impact of surveillance.35 HCPs were categorised as either a PCP (internal medicine, family practice) or gastroenterologist/hepatologist. Over three years, 17% of patients had regular surveillance (annual USS ± AFP), 38% had inconsistent surveillance (USS ± AFP on one or more occasion), and 55% had no surveillance prior to HCC diagnosis. Patients seen by a gastroenterologist alone or in combination with a PCP were fivefold more likely to undergo surveillance.35 Another retrospective study of PCPs and gastroenterologists showed patients managed by gastroenterologists were more likely to receive surveillance.39 Failure to undergo surveillance was attributed to PCPs or patients not following gastroenterologist recommendations; failure in discharge planning and communication to a patient’s PCP; and a diagnosis of cirrhosis made by a non-PCP specialist without a further surveillance recommendation.39 In a study using retrospective VA data,42 the strongest predictor of consistent surveillance was patient management by gastroenterologists, hepatologists or infectious diseases physicians.
Similarly, a US study reported just 2% of patients received consistent surveillance, 33% received inconsistent surveillance and 65% had no surveillance.41 Surveillance was associated with gastroenterology/hepatology subspecialty care (OR 1.88; 95% CI: 1.44, 2.46).41 Another US-based study reported that over three years just 2% of patients had six or more USS; 26% had three or more USS; 77% of patients underwent one or more USS; and 94% had AFP levels taken on one or more occasion.40 For surveillance-detected HCC, 70% were diagnosed at early stages, compared with 40% of non-surveillance cases. In turn, 23% of surveillance-detected HCC patients were eligible for curative treatment, whereas no patients in the non-surveillance group were.40
A study using a VA dataset44 showed living further away from medical centres was negatively associated with surveillance. In subgroup analyses, patients living >48 km from medical centres were more likely to use non-VA services for USS/AFP. Therefore, these patients might have been engaged in surveillance, but not captured in the data.
HCP-focused studies
A qualitative study on PCPs providing care to CHB and chronic hepatitis C (CHC) patients reported facilitators of surveillance including receiving follow-up care within their communities, other community members being supportive of the HCP and patient navigators of the same ethnicity.24 Barriers included cost, patients being too busy with work commitments, HCPs’ and patients’ lack of knowledge regarding CHB, CHC and indications for HCC surveillance, language and cultural barriers.
In another qualitative study,21 focus groups with 19 HCPs (PCPs, gastroenterologists and infectious diseases specialists) working with CHB patients identified knowledge, motivational and technical/logistical barriers. Knowledge barriers included a lack of awareness of HBV infection status and recommendations for surveillance, misunderstanding of serology results and communication failures between PCPs and other specialists. Motivational barriers included a lack of confidence in the utility of HCC surveillance, limited evidence regarding survival benefits and surveillance being of low priority for patients with multimorbidity. Technical and logistical challenges included navigating the health system, unclear HCP roles and a lack of protocols for patient follow-up. Patient barriers, as reported by HCPs, included costs, active substance use and multimorbidity.
Six cross-sectional studies20,22,23,25,51,53 used surveys to evaluate barriers and facilitators of surveillance for HBV and hepatitis C virus (HCV) patients. The main barrier identified was a lack of HCP knowledge of HCC surveillance recommendations.20,22,23 Other HCP-related barriers were a lack of resources and difficulty accessing specialty care (ie gastroenterologists/hepatologists). HCPs also cited patient financial constraints as an important barrier.22,25
Regarding surveillance approaches, another study of PCP surveillance practices reported that effective surveillance can be incorrectly conducted by monitoring liver enzymes (30%) or by conducting a clinical examination (11%).52 Other factors impacting surveillance included competing priorities within the clinical setting and patient financial constraints.25,49
Discussion
This is the first systematic review of the evidence for HCC surveillance in primary care settings. We found that irrespective of HCC risk factors, surveillance rates were consistently lower for patients managed by PCPs compared with gastroenterologists or hepatologists. When additional support was provided to PCPs to address a range of barriers, surveillance rates increased substantially.
HCC surveillance is of critical importance in reducing morbidity and mortality for high-risk populations. For CHB patients with cirrhosis, the annual risk of developing HCC is 3–5%,54,55 and 0.42% in the absence of cirrhosis.56 For alcohol-related cirrhosis, the annual risk is 2.5%,57 and for non-alcoholic steatohepatitis (NASH) it is 2.6%.58 The HCC progression probability can be reduced through antiviral medications for CHC and CHB, lifestyle modifications for alcohol- and NASH-related cirrhosis and engaging high-risk patients in surveillance.59 Primary care is the ideal setting for this because it is usually the first point of contact patients have with the health system. Understanding how primary care can be supported to undertake surveillance in line with recommendations is of critical importance in reducing morbidity and mortality of HCC.
All studies evaluating interventions to support PCPs reported improved surveillance rates. These interventions involved the provision of support, such as software-based clinical management systems,33,38 PCP training,37 support staff following up with patients and HCPs19,34 and mailed outreach with patient navigators.43 Surveillance programs can be resource intensive. This was noted in one study in which staff reported the increased workload associated with surveillance was not sustainable.34 Therefore, an effective and equitable surveillance approach must be adequately resourced to ensure sustainability.
We observed substantially different HCC surveillance rates in non-interventional studies, ranging between 2% and 73%. Selection bias likely impacts these rates; surveillance rates tended to be higher in viral hepatitis studies set in high-HCC-prevalence clinical settings, with high rates of CALD and uninsured patients. For studies focused on cirrhotic patients, surveillance rates tended to be higher than observed in other studies. This difference was driven by surveillance rates reported for all HCPs combined. Gastroenterologists and hepatologists typically have higher surveillance rates among their patients compared with PCPs, which contributed to higher overall rates of HCP surveillance.
Barriers to HCC surveillance included lack of knowledge and awareness of HCC and associated risk factors. A key barrier identified was PCP lack of awareness of surveillance recommendations. Many professional societies have published HCC surveillance recommendations.11,60 These recommendations are critically important to support clinical decision making; therefore, awareness of these guidelines and improving knowledge of HCC and relevant aetiologies must form part of a strategy to improve HCC surveillance in primary care settings.
Another important barrier might be the time factor. PCPs provide care to patients with multiple acute and chronic health conditions and are frequently time constrained.61,62 In this context, prevention strategies, such as HCC surveillance, can become a low priority. Supporting PCPs to conduct surveillance plays an important role in primary care-based programs.
PCPs noted complex health systems contributed to lower rates of surveillance. Health system barriers identified included patient eligibility regarding health insurance, clinical information not shared between HCPs, lack of clarity and protocols regarding HCP roles for patient follow-up.
No patient-reported data were identified in this review. Instead, patient barriers were reported by HCPs. An important barrier was the costs patients incur when undergoing surveillance, including for USS, MRI, CT and other related investigations. This was a barrier cited in US-based studies, where universal health insurance does not exist. Another barrier was related to many high-risk CHB patients being of migrant backgrounds, which contributed to difficulties in navigating health systems and language and cultural barriers. This is consistent with a survey of cirrhotic patients (ineligible for this review), which identified barriers in navigating healthcare systems such as difficulty scheduling USS, costs and transportation difficulties.63 In addition, fear of a cancer diagnosis and time commitments to undergo surveillance were reported.63 However, patient knowledge that cirrhosis is a high-risk for HCC was associated with receipt of surveillance. In our review, we found that facilitators of HCC surveillance were focused on community aspects, including patients preferring to receive follow-up care within their communities, positive regard of the HCPs from other community members and patient navigators of the same ethnicity.
Although HCC survival is associated with earlier tumour detection and improved survival, it is also important to note that patients can experience surveillance-related harms. In a study of 680 cirrhotic patients undergoing USS ± AFP, 28% of patients with false-positive results experienced harm,40 defined as subsequent CT and MRI scans and liver biopsies. Harm can occur from radiation exposure and contrast injuries associated with CT and MRI scans,64,65 and bleeding and tumour seeding from liver biopsy.66 In addition, the patient and/or health system incur financial costs. Importantly, no evidence has been published regarding how patients experience these harms or how surveillance impacts patients’ quality of life.53
The limitations of this study are largely related to the quality of evidence that was included, with most studies based on analyses of retrospective cohorts or cross-sectional surveys. The generalisability of these results is impacted by selection biases within the included studies, and these results need to be interpreted with caution. Due to the heterogeneity of the studies included, a meta-analysis was not performed.
Conclusions
This systematic review found that primary care-based HCC surveillance programs are most likely to be effective if PCPs are supported (eg recall systems, clinician education or reminder systems), programs are of low or no cost to patients, abdominal imaging and pathology are easily accessible and information is communicated to all treating HCPs and patients in a timely manner.