The most common sources of iatrogenic pain in childhood are needles used for routine immunisations;1 the associated pain is distressing for children, their parents and healthcare providers.2 One in four adults is estimated to have a fear of needles,3 which often develops in childhood.4 If immunisation pain and distress are poorly managed, this can result in anxiety prior to future medical procedures, and healthcare avoidance behaviour.5 It has been reported that up to 10% of the population avoids vaccination due to needle phobia.5
If all recommended vaccines are administered, an Australian child will have received nine needles prior to their first birthday, and another five in their second year of life. As the child matures, their ability to conceptualise and react to the prospect of a painful experience increases. The four-year-old immunisation is often challenging for all involved: the child, the parents and those administering the vaccine.
Various techniques are recommended to reduce the pain and distress associated with immunisation. These include optimising positioning and injection techniques, tactile stimulation, topical anaesthesia and various distraction techniques.2 This study sought to determine whether the use of a virtual reality (VR) intervention is superior to standard care for young children undergoing vaccination.
VR is a computer system that allows users to be immersed in and explore an interactive three-dimensional environment.6 In recent years there has been increased access to the technology, with it now being available through the purchase of an inexpensive headset and the use of a smartphone. VR has been successfully used in various settings where children might experience painful procedures, including application of burns dressings,7–12 dental treatment,13 intravenous cannula placement14 and medical oncology treatments.15,16
Although VR appears to be safe and effective for the management of pain, the underlying mechanism of action is yet to be fully elucidated.17 It has been suggested that a patient’s attention is directed into a virtual world, leaving less attention available to process incoming signals from pain receptors.18 Other effects on pain perception relate to emotion, concentration and memory.19
Objective
The aim of this study was to determine the effectiveness of VR content delivered through a headset in reducing pain and distress associated with four-year-old childhood vaccination within a general practice setting. The hypothesis was that VR content delivered through the headset would reduce pain and distress associated with childhood vaccination compared with standard techniques used in the primary care setting.
Methods
Trial design and participants
This was a randomised controlled superiority trial comparing the effectiveness of VR against standard care for the pain and distress associated with four-year-old vaccinations within a general practice setting. The trial was conducted in a single general practice within metropolitan Melbourne, which provides routine vaccinations to approximately 120 children aged four years per year. The detailed trial protocol has been published previously.20
To be eligible for inclusion in the study, children had to be attending the general practice for their four-year-old immunisations and judged by their treating doctor to be able to comply with the study protocol for its duration. Written informed consent, signed and dated by the parent/legal guardian according to local regulations, was obtained.
Children were excluded if a significant medical disease or condition was present that was likely to interfere with the child’s ability to participate in the study and the parent/legal guardian was unable to provide informed consent.
The parents/legal guardians of children attending the clinic for their routine immunisations were approached to participate in the study. During routine review by a doctor prior to vaccine administration, each child was assessed for eligibility against the study inclusion and exclusion criteria. If eligible, a verbal explanation of the study was provided to the parent/legal guardian by the treating doctor and any questions were answered at that time. Once the child was moved to the procedure room, nursing staff demonstrated the VR equipment and sought assent from the child and formal written consent from the parent/guardian.
Randomisation and masking
Children were randomly assigned to receive either VR or standard care using blocked randomisation, with the block sizes randomly varying from four to eight. This allocation sequence was generated by a study author (SC) using computer-generated random numbers. This occurred independently of any staff involved in the recruitment of patients for the study, and SC did not recruit any patients into the study. Allocation was concealed in opaque study envelopes, which were opened once the child’s parent/legal guardian had signed the consent forms. Due to the nature of the study intervention, it was impossible to blind patients, parents/legal guardians or observers to the allocated intervention.
Interventions
The study intervention was VR digital content that was delivered through a purpose-designed headset, containing a Google Pixel XL and Google Daydream VR headset hardware unit (Google, Mountain View, CA, USA). The unit is used as a form of VR apparatus diversion therapy device (Therapeutic Goods Administration-approved class 1 medical device [ID 156474]), manufactured by Smileyscope Pty Ltd (Melbourne, Vic, Australia). The purchase of this device was at a recommended retail price using research grant funding, and the company had no role in protocol design, patient recruitment, data analysis or the decision to publish any findings.
The intervention group received the VR intervention prior to vaccine administration in addition to standard care, whereas the control group received standard care alone. The VR headset played an interactive marine adventure that starts with a relaxation sequence and progresses to underwater scenes, including gaze-based tracking of virtual fish. The same VR intervention, which lasted for one minute was provided to all children, with the injection provided approximately 30 seconds after the commencement of the sequence.
Standard care within the clinic includes a range of interventions to reduce the pain and distress of immunisations. These interventions include distraction through conversation about age-appropriate interests, such as pets, siblings or an upcoming birthday, reading a book or watching a video on a parent’s phone. The interventions in the standard care group were documented. Topical anaesthetics are not routinely offered by the clinic.
Outcome measures
The primary end point for this study was the difference in self-rated pain scores between the intervention and control groups. Different self-report measurement tools are recommended for different age groups, consistent with the expected verbal and emotional level attained. The Faces Pain Scale – Revised (FPS-R) is recommended for those aged 4–16 years21 and was the primary outcome measure used in the study. The FPS-R shows a series of six faces, ranging from a happy face at 0, or ‘no hurt’, to a crying face at 10, which represents ‘hurts like the worst pain imaginable’.21
To allow for the likely range of developmental abilities in normal children aged four years, the poker chip tool22 was used as a secondary outcome measure for self-reported pain. This tool, recommended for children aged between three and six years, quantifies pain intensity by using four objects as counters, or poker chips, to represent amounts of pain. Children indicate how much hurt they have by referring to one counter as a little bit of hurt, two as a little more hurt, three as more yet and four as the most hurt they could ever have.23
Other secondary outcome measures include observer ratings of pain and distress by parents/legal guardians and healthcare providers and the overall satisfaction of parents/legal guardians with the experience (VR and immunisation). These were recorded on standard 100-mm visual analogue scales (VAS). Adverse effects relating to the use of VR intervention (eg motion sickness) were recorded as free text.
Other data collected prior to immunisation, through a questionnaire administered to the parent/legal guardian, were: child’s age, sex, previous exposure to VR, languages spoken at home other than English, significant medical history, visual, behavioural or developmental concerns and level of apprehension towards needles (rated as low, medium or high). Following the procedure, outcome data were collected and recorded on a study case report form. The outcome data included distraction and other interventions used, the child’s experience of the needle (the poker chip tool and FPS-R), observed pain and distress (parent/legal guardian), observed pain and distress (healthcare provider) and any adverse effects of VR (eg motion sickness). The proceduralist recorded their previous experience with vaccine administration (<10, 10–50, 51–100, >100 childhood vaccines administered) and the patient and parent/legal guardian were asked to rate their overall enjoyment (low, medium or high) of the immunisation experience.
Statistical analysis
Sample size was determined using GraphPad Statmate (V.2.0 for Windows; GraphPad Software, San Diego, CA, USA). Forty-two children in each arm of the study would be able to demonstrate a difference of two points in the 10-point FPS–R score, with the use of an unpaired t-test with a power of 0.8, an a of 0.05 and a standard deviation (SD) of 3.2. The SD of 3.2 is a conservative estimate based on previous validation of the FPS-R in children aged four to six years in the hospital setting.21 A difference of two points (one face) is considered the minimum clinically significant difference in the FPS-R.24 To allow for any attrition due to inability to comply with the VR treatment, the study sample size was increased to 100 in total, with recruited children randomised 1 : 1 to receive VR or standard care.
The primary analysis was performed according to the intention-to-treat principle. Continuous variables are expressed as the median with interquartile range (IQR) and were compared using the Wilcoxon rank-sum test. Categorical data are presented using numbers and percentages and were analysed using the Chi-squared or Fisher’s exact test, as appropriate. Two-sided P<0.05 was considered to indicate statistical significance. Statistical analyses were performed using Stata version 16 (StataCorp, College Station, TX, USA).
Exploratory subgroup analyses were used to compare results between the following groups: (1) those with versus without previous exposure to VR; and (2) those with a parent-rated high level of apprehension towards needles versus those with a low or medium level of apprehension towards needles.
Ethics
Ethics approval was obtained from the RACGP National Research and Evaluation Ethics Committee (NREEC 18-010). The trial was registered with the Australian and New Zealand Clinical Trials Registry (trial registration number ACTRN12618001363279).
Results
During the study period (June 2019 – February 2020), 100 children were assessed for study eligibility (Figure 1) and 87 completed the study. Study enrolment ceased in early 2020, prior to completing the planned enrolment of 100 children, due to the impact of the COVID-19 pandemic.
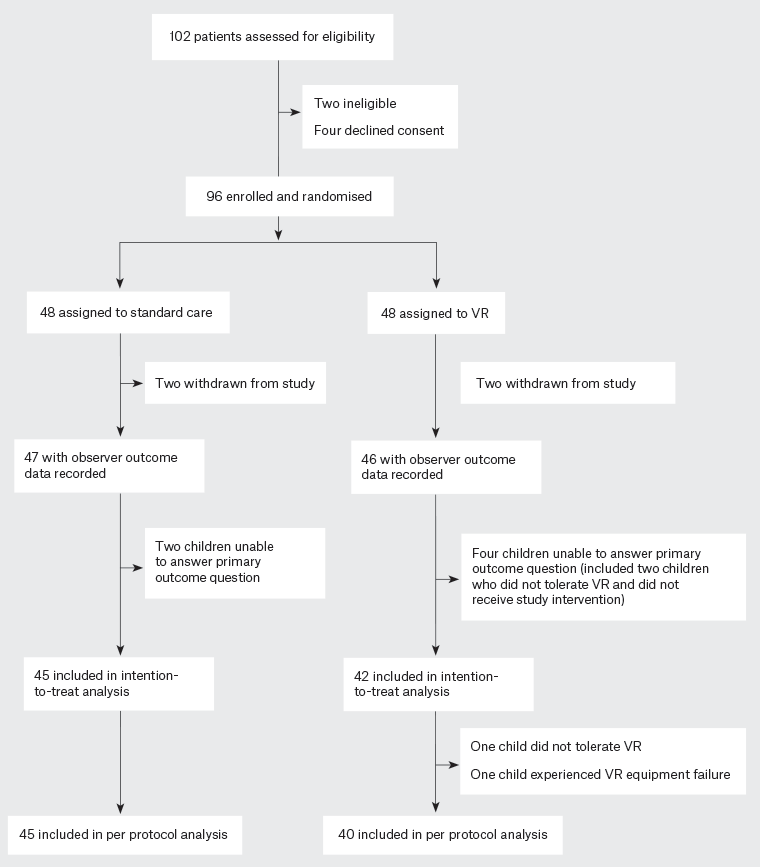
Figure 1. CONSORT diagram. Click here to enlarge
VR, virtual reality.
The CONSORT diagram for the study is shown in Figure 1. As shown in the figure, 96 children were randomised; three were withdrawn from the study after randomisation (in one case because the parent wanted VR and in two cases because the child did not want to put the headset on). Six children who were randomised to the study were not able to provide an answer to the primary outcome (FPS-R): two in the standard care group and four in the VR group (including two who refused VR and did not receive the study intervention). Thus, 87 children were included in the intention-to-treat analysis. Two children from the VR group did not receive the study intervention: one did not tolerate the VR intervention and, for the other child, the VR equipment failed to operate.
The baseline characteristics of those eligible for the intention-to-treat analysis are presented in Table 1. The median age of enrolled children was 48 months. Most children spoke English as a primary language, and most had not previously been exposed to VR. Those randomised to standard care were more likely to be male, whereas those randomised to VR were more likely to be female.
| Table 1. Baseline characteristics of the children included in the study |
| |
Standard care (n=45) |
VR intervention (n=42) |
| Age (months) |
48 [48–49] |
48 [48–49] |
| Female sex |
19 (42.2) |
23 (56.1) |
| Primary language – English |
43 (95.6) |
39 (92.9) |
| Previous VR experience |
6 (13.3) |
2 (4.8) |
| Needle apprehension |
2 [1–3] |
2 [1–2] |
| Vision concerns |
0 (0) |
1 (2.4) |
| Behavioural concerns |
1 (2.2) |
1 (2.4) |
| Developmental concerns |
1 (2.2) |
0 (0) |
| Medical historyA |
5 (11.1) |
4 (9.5) |
Data are presented as the median [interquartile range] or n (%).
VR, virtual reality.
AMedical history included anaphylaxis/allergy (n=3), asthma (n=2), and tympanostomy tubes, liver transplant, meningitis, prematurity (n=1 of each). |
There was no difference in child ratings of pain and distress (Figure 2). However, significant differences in favour of VR were found when comparing perceptions of pain and distress from both parents and observing nursing staff (Table 2; Figure 3). The VR intervention was rated as more enjoyable than standard care.
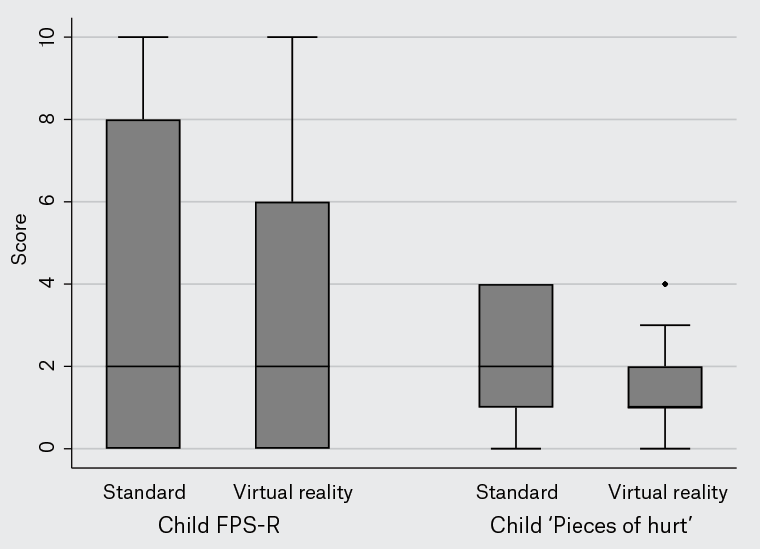
Figure 2. Child-rated pain and distress.
Children were asked to rate their pain and distress using the Faces Pain Scale – Revised (FPS-R) and the poker chip scale. The FPS-R presents children with six faces, ranging from a happy face at 0 (‘no hurt’) to a crying face at 10 (‘hurts like the worst pain imaginable’). The poker chip scale quantifies pain intensity by using four objects as counters, or poker chips, to represent amounts of pain: one counter indicates a little bit of hurt, two a little more hurt, three more hurt and four the most hurt they could ever have. The boxes show the interquartile range, with the median value indicated by the horizontal line. Whiskers show the range; circles indicate outliers.
| Table 2. Pain, distress and enjoyment scores |
| |
Standard care (n=45) |
VR intervention (n=42) |
P-value |
| Child rating of pain/distress |
|
|
|
| Faces Pain Scale – Revised score |
2 [0–8] |
2 [0–6] |
0.11 |
| Poker chip tool scoreA |
2 [1–4] |
1 [1–2] |
0.24 |
| Observer ratings of pain/distress |
|
|
|
| Parent rating of pain (VAS) |
23 [7–70] |
11 [1–28] |
0.01 |
| Parent rating of distress (VAS) |
27 [4–78] |
4 [0–28] |
0.001 |
| Practitioner rating of pain (VAS) |
22 [10–65] |
11.5 [4–21] |
0.005 |
| Practitioner rating of distress (VAS) |
23 [7–75] |
9.5 [0–21] |
0.002 |
| EnjoymentB |
1 [1–2] |
2 [2–3] |
<0.001 |
Unless indicated otherwise, data are presented as the median [interquartile range]. P-values were calculated using the Wilcoxon rank-sum test.
AData regarding the poker chip tool in the virtual reality (VR) group were only available for 41 children.
BData regarding enjoyment in the standard care group were only available for 37 children.
VAS, visual analogue scale. |
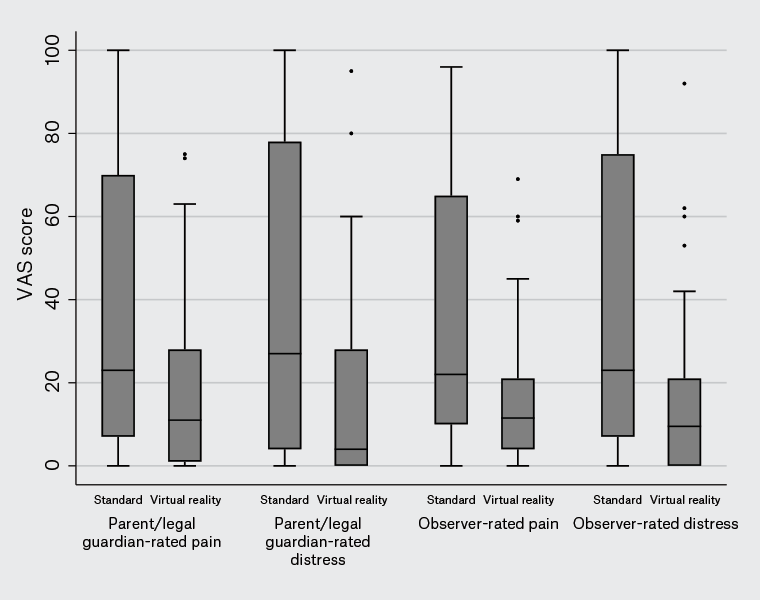
Figure 3. Parent/legal guardian- and observer-rated pain and distress in children receiving immunisations, recorded on a standard 100-mm visual analogue scale (VAS).
The boxes show the interquartile range, with the median value indicated by the horizontal line. Whiskers show the range; circles indicate outliers.
Per-protocol analysis (85 patients; Appendices 1,2; available online only) yielded similar results. We conducted planned exploratory subgroup analysis on children with (n=8; Appendices 3,4; available online only) and without (n=79; Appendices 5,6; available online only) previous exposure to VR, and those with (n=25; Appendices 7,8; available online only) and without (n=61; Appendices 9,10; available online only) high levels of needle apprehension. No subgroup analysis demonstrated a benefit of VR over standard care for child-rated outcomes. However, parent and practitioner ratings of pain and distress favoured VR over standard care among those without previous exposure to VR, and among those with low or medium levels of needle apprehension. No difference between VR and standard care was observed among those with previous exposure to VR and among those with high levels of needle apprehension; however, it is likely that these analyses were underpowered due to the small sample sizes. Enjoyment was higher in the VR than standard care group for all subgroups, apart from those with previous exposure to VR (eight children).
No adverse events relating to VR or immunisation were reported in either group. Two children randomised to VR removed the goggles before their immunisation was administered.
Discussion
The results of this study suggest that VR might be a useful intervention for children aged four years undergoing routine childhood immunisations. Pain and distress ratings by observers (both parents/legal guardians and health practitioners) significantly favoured VR, and patient/family ratings of enjoyment favoured VR. However, our primary outcome, a self-rated pain score applied by the child, did not show any difference between the VR intervention and standard care.
Pain and distress are subjective experiences, felt differently by every person. Therefore, best practice research relies on self-reported measures. However, although we included self-rating pain scales suitable for the population included in our study (children aged four years), these children are at the lower end of the validated age scale and had not used the scale before. It is notable that six children (two in the standard care group and four in the VR group) were unable to provide an answer to the primary outcome. It is unclear whether children using such a pain scale for the first time can provide a reliable self-rating. A systematic review of self-reported pain measures, available after our clinical trial protocol had been registered, was unable to recommend any self-reported pain scale for children aged six years.25 This suggests that ratings from parents/legal guardians and observers might be more reliable than those from young children.
Given that parents make all medical decisions on behalf of their young children, the fact that their ratings favoured the VR intervention (in terms of less distress, less pain and more enjoyment) suggests that they would be in favour of using VR for children aged four years undergoing routine immunisations. Despite this, the relatively high cost of the proprietary VR headset and software (over A$2000 per year as of August 2022) and the additional time required for staff training to maintain, charge and clean the VR equipment and to explain how to use the headset on each occasion might prove to be barriers to widespread adoption.
A major limitation of this study is the unblinded nature of the intervention. Due to the nature of the VR intervention, it was impossible to blind the parent/legal guardian, child or clinician observer as to whether the VR intervention had been applied. This might have biased responses towards VR, particularly if there was a pre-existing bias in favour of the intervention. It is possible that such a bias existed, illustrated by one parent withdrawing their child (after randomisation) because they were allocated to standard care.
The inclusion of only children who were judged by their treating doctor to be able to comply with the study protocol might have introduced some selection bias, and therefore limits generalisability. However, this was a pragmatic decision that we believe reflects the practical realities of the general practice setting.
In conclusion, we did not demonstrate a difference in self-rated pain and distress by children aged four years receiving routine immunisations when comparing standard care to a VR intervention. However, parent/legal guardian and observer ratings of pain and distress, as well as overall ratings of enjoyment, all favoured VR.