Australian general practitioners (GPs) excise an increasingly large number of suspected malignant skin lesions.1 These excisions tend to be performed in treatment rooms, involve a circumferential incision <5 cm, use local anaesthetic agents and are closed with simple or continuous sutures.
A common anaesthetic used for these procedures is lignocaine (also known as lidocaine and sold under the brand name Xylocaine).2 Lignocaine is often co-administered with adrenaline, which induces vasoconstriction, decreasing bleeding and prolonging the analgesic effect by slowing systemic absorption.2
Reported complication rates for these procedures are variable, with Heal et al3,4 finding rate of infection alone to be 8.6% and Botting et al5 reporting a 2% rate for any complication. The most common complications are wound dehiscence and/or infection.3–6 Significant risk factors for such complications are well described in the literature and include patient age >80 years and excisions on the limbs.4,7–10 Although anaesthetic dose is reported as a risk factor for systemic complications such as tachycardia, it is not discussed with regard to wound healing in dermatological surgery settings.4,7–14
Although no studies describe lignocaine dose as a risk factor for complications associated with wound healing, the effect of lignocaine on wound healing has been thoroughly evaluated by in vivo animal studies. These studies have been unable to find a consistent effect for lignocaine.15–20 For example, Morris et al concluded that increasing concentrations of both lignocaine and adrenaline independently corresponded to decreased tensile strength, thus negatively affected wound healing.15 By comparison, Rodrigues et al found that lignocaine temporarily impaired collagen arrangement and the inflammatory process, but these effects did not persist at 21 days.19 In summary, research examining complications after skin cancer excisions does not consider lignocaine as a risk factor, whereas wound healing research is inconclusive with regard to the effects of lignocaine.
This study was prompted following anecdotal observation by nursing staff of a rural Victorian GP clinic that high doses of lignocaine administered during skin lesion excision corresponded to increased complications. The study’s objective was to evaluate whether higher doses and/or volumes of lignocaine were associated with increased complication rates after skin cancer excisions, and whether using reduced doses of lignocaine corresponded to increased episodes of inadequate analgesia.
Methods
Overview
This pilot study initially consisted of a retrospective audit of an initial time period (henceforth called the pre-intervention period). Analysis of data collected during this period showed a positive association between lignocaine dose and complication rates. This information was presented and discussed at a roundtable of practitioners at the clinic. A conclusion of this discussion was the collection of post-intervention data to identify changes in lignocaine dose and/or volume and any complication rates (the post‑intervention period).
Ethics approval for the study was granted by the Deakin University Human Research Ethics Committee (DUHREC; Approval no. 2022-177).
Participants and data collection
A file review was completed in the clinic’s electronic medical records (ZedMed) of Medicare item numbers billed for skin cancer and presumed skin cancer excisions (Items 31356–31376) for all doctors for the pre-intervention period (14 October 2019 – 5 November 2020 and the post-intervention period (1 February 2021 – 17 February 2022). Electronic records were cross-checked with hard-copy documentation.
Data extracted were patient demographics, lesion location, benign/malignant status, histopathological diagnosis, treating doctor and their stages of training. Infective complications were defined by documentation of ‘infection’, ‘pus from wound’, ‘cellulitis’, or if there was prescription of antibiotics. Dehiscence was defined by documentation of ‘dehiscence’, ‘wound gaping’ or if resuturing was required after the planned removal of sutures. Lesions reporting both dehiscence and infection were reported as infection. This was chosen because a major risk factor for wound dehiscence is surgical site infection.21 Dose was calculated as volume (mL) multiplied by concentration (mg/mL). Patient and lesion characteristics are shown in Table 1. All excisions were performed using lignocaine with adrenaline.
| Table 1. Patient and lesion characteristics |
| No. excisions |
443 |
| Pre-intervention |
149 |
| Post-intervention |
294 |
| Sex (n) |
| Male |
254 |
| Female |
189 |
| Age (years) |
| Mean |
66.41 |
| Median |
69 |
| Range |
21–96 |
| Histology (n) |
| Aesthetic |
4 |
| Basal cell carcinoma |
142 |
| Melanoma |
31 |
| Squamous cell carcinoma |
82 |
| Solar keratosis |
67 |
| Other |
117 |
| Location (n) |
| Head/neck |
136 |
| Torso |
164 |
| Limbs |
143 |
| Other |
0 |
| Clinician experience (n) |
| Registrar |
133 |
| Consultant |
310 |
| Complication rate (%) |
| Pre-intervention |
13.40 |
| Post-intervention |
7.50 |
| Combined period |
9.50 |
| No. complications |
42 |
| Pre-intervention |
20 |
| Post-intervention |
22 |
| Type of complication (n) |
42 |
| Infection |
28 |
| Dehiscence |
14 |
Educational event
In December 2020, the results from the initial audit were presented to the nurses and doctors involved in patient care at the facility. These initial results showed a significant relationship between lignocaine dose and complications. These practitioners hypothesised that the dose and volume of lignocaine affected complications rates, and hence deliberated methods for reducing lignocaine used in procedures. Suggestions included reducing the overall dose administered by infiltrating lower-concentration solutions either by dilution or using premixed products.
Statistical analysis
Our data was not normally distributed according to the Shapiro–Francia test for normality (P>0.25). Hence, group differences were determined using the Kruskal–Wallis test. Data are reported as the mean, median, range and standard deviation (SD), where appropriate. In all cases, P<0.05 was considered significant. Power calculations were not performed. Statistical analysis was conducted using STATA version 17 (StataCorp, College Station, TX, USA).
Results
After the intervention there was a 43% reduction in the dose of lignocaine administered and a 25% reduction in the volume administered. Complete comparison of dose and volume between periods is given in Table 2.
| Table 2. Lignocaine doses and volumes |
| |
Mean±SD |
Range |
% change |
P-value for change |
| Dose (mg) |
| Pre-intervention |
116.13±69.84 |
6–300 |
–43 |
<0.001 |
| Post-intervention |
65.79±54.25 |
2–250 |
|
|
| Volume (mL) |
| Pre-intervention |
5.97±3.35 |
0.3–15 |
–25 |
<0.001 |
| Post-intervention |
4.46±3.58 |
0.2–20 |
|
|
| SD, standard deviation. |
Excisions from 13 different clinicians were included in the study; however, doctors performed variable numbers of excisions. For example, one practitioner performed 54% of all procedures, whereas six performed fewer than 1% of procedures.
There were 42 complications, of which 20 occurred during the pre-intervention period and 22 occurred during the post-intervention period. There was a significant positive relationship between the complication rate and the lignocaine dose in both the combined and pre-intervention periods, but no significant relationship in the post-intervention period.
There were no significant relationships between the complication rate and patient gender, patient age >80 years (extremes of age >80 and <10 years are identified as a significant risk factor in this systematic review; however, those aged <18 years were excluded from the study for ethical reasons) and whether a practitioner was a consultant or registrar. However, lesions either located on the limbs or malignant upon histology were found to have a significant positive relationship with the complication rate.
The dose of lignocaine was found to have a significant positive relationship with both the complication rate and dehiscence in both periods combined and during the pre-intervention period, but not during the post-intervention period.
The volume of the lignocaine solution had a significant positive association with both the complication rate and dehiscence during the combined and pre-intervention periods, but not in the post-intervention period. Lignocaine dose or volume were not significant risk factors for infection in either the pre- or post-intervention period.
Discussion
The results of this study demonstrate a positive relationship between both lidocaine dose and volume and the complication rate after excision of suspected skin malignancies in general practice. This represents a unique contribution to the literature because previous research into complications after skin cancer excision has not considered local anaesthetic as a contributor to wound healing.4,7–14 Furthermore, it suggests that minor procedural changes could reduce the complication rate. Skin malignancies are Australia’s most common cancer, accounting for over one million GP presentations per year and for over one-tenth of total cancer expenditure.22–24 Consequently, any intervention that reduces complications associated with the excision of skin malignancies will alleviate clinic workload and healthcare burden more broadly.
Of note, infiltrated volume was significantly positively associated with the complication rate and dehiscence in the present study (Figure 1). Theoretically, a larger volume of injected fluid could increase dehiscence by distorting wound architecture, a hypothesis also posited by Morris and Tracey,15 who attributed a portion of their observed impaired wound healing to volume alone. Consequently, injecting a higher volume of more dilute local anaesthetic (as proposed by clinicians during the educational event) may increase rather than decrease the complication rate. Although volume and dose were significant factors for complications and dehiscence, neither was a significant factor for infection. This suggests that it is the observed significant effects of dose and volume on dehiscence that contribute to the significant positive relationship with the complication rate. There was no significant relationship between dehiscence and the volume of lignocaine injected after the intervention; this could be attributed to the reductions in lignocaine dose and volume as a result of changes in clinician behaviour that led to only four wounds dehiscing over this period, thus limiting statistical power. The relationship between volume, dehiscence, and the complication rate over six-monthly intervals is shown in Figure 1. Nonetheless, these results suggest that educational interventions can reduce the dose and volume of lignocaine administered, as well as complication rates, without leading to episodes of inadequate analgesia.
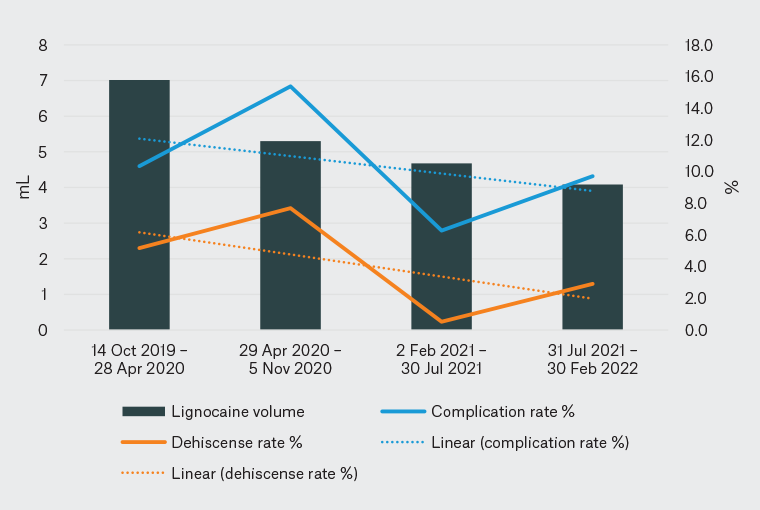
Figure 1. Six-monthly analysis of lignocaine volume versus complication and dehiscence rates.
The results of this study indicate that malignant lesions and those located on limbs were at higher risk of infection or dehiscence (Table 3). This is consistent with a systematic review by Delpachitra et al.7 Although not in the scope of this paper, these findings may justify risk-reduction strategies such as prophylactic antibiotics.
| Table 3. Significance of the relationship between lignocaine dose and volume and the rate of complications |
| |
P-value |
| Complication risk factorsA |
| Patient gender |
0.3457 |
| Clinician seniority |
0.3451 |
| Patient age >80 years |
0.8952 |
| Lesion on limb |
0.0248 |
| Malignant lesion |
0.0242 |
| Dose vs complications |
| Pre-intervention |
0.0274 |
| Post-intervention |
0.1994 |
| Combined period |
0.0084 |
| Dose vs infection |
| Pre-intervention |
0.486 |
| Post-intervention |
0.2048 |
| Combined period |
0.2233 |
| Dose vs dehiscence |
| Pre-intervention |
0.0098 |
| Post-intervention |
0.7263 |
| Combined period |
0.0030 |
| Volume vs complications |
| Pre-intervention |
0.0303 |
| Post-intervention |
0.2806 |
| Combined period |
0.0209 |
| Volume vs infection |
| Pre-intervention |
0.4267 |
| Post-intervention |
0.2697 |
| Combined period |
0.2466 |
| Volume vs dehiscence |
| Pre-intervention |
0.0188 |
| Post-intervention |
0.8312 |
| Combined period |
0.0136 |
| AThese factors represent the significance of those variables in explaining change in complication rates. |
Limitations
The clinical attributability of these results is limited by a variety of factors, including the expected challenges of retrospective audits in primary care environments and the methodological shortcomings of this research specifically.
For example, retrospective review of documentation meant that of 786 excisions, only 443 were suitable for inclusion in the analysis. The most common cause for exclusion was finding no corroboration of an excision in records or incomplete dose data. When there was no documentation of a complication it was assumed that no complication occurred. Reassuringly, any confounding effect of this decision is diminished by there being no significant difference in complications between the included and excluded lesion groups (P=0.4208). In addition, the post-intervention dataset contained two-thirds of the total excisions despite being seven days shorter. However, the smaller number of lesions excised in the pre-intervention period can be partially attributed to the reduction of skin checks during Victoria’s COVID-19 restrictions, which has been reported in other research.25
The research may be confounded by the methodological limitations of the study; most prominently, the research was non-randomised, was performed at a single clinic and some authors were clinicians, partners or students at the clinic. This may be a form of the Hawthorn effect, whereby clinician awareness of observation introduces bias from defensive behaviour change (eg laying additional sutures).26 Furthermore, a single site makes comparison with climactically distinct locations less reliable, given humidity has been recognised elsewhere as a contributor to wound healing.27
Factors such as patient age, patient sex, clinician experience and lesion location and histopathology were accounted for, with no significant difference in lesion characteristics between the pre- and post-intervention periods (Table 4). However, other potential risk factors for complications in minor dermatological surgery, such as diabetes, were not included.4,8,9 Nonetheless, the systemic review mentioned previously by Delpachitra et al found that underlying comorbidities such as diabetes and smoking status were not significantly related with complications, rather the only consistently significant risk factors were below-knee location, re-excision of skin cancer, extremes of age and whether the lesion was malignant.7 As such, not identifying potential risk factors including diabetes or smoking status might not have had a substantial effect on reported outcomes.
| Table 4. Significance of differences between the pre- and post-intervention periods |
| |
P-value |
| Patient gender |
0.4686 |
| Patient age |
0.082 |
| Malignant lesion |
0.876 |
| LocationA |
0.5328 |
| Clinician seniority |
0.4312 |
| ALesions located on extremities. |
Of note, although most excisions included were of similar size, there inevitably was some variability. Attempts to estimate the surface area of excisions or extrapolating based on the number of sutures was unsuccessful with the available data. It is intuitive that the larger lesions would be more likely to dehisce than smaller lesions, and thus may contribute to variance in results.
Despite the authors’ efforts to control for possible variables, this research is limited compared with more rigorous investigation. Nevertheless, it has identified a framework for future research that is described below.
What comes next?
The methodological limitations of this audit mean the results are best interpreted as a pilot study for a prospective randomised control trial. Alternatively, an interrupted time series analysis could be considered. Regardless, any future study should examine the hypotheses that injected the volume and dose of lignocaine increase the risk of wound dehiscence specifically and the rate of wound complications generally. Any future study should involve multiple clinics to minimise site- and clinician-specific factors. Patients could be randomly allocated for standardised doses of lignocaine to be delivered in different concentrations, and thus different volumes. In addition to the results and variables from this pilot study, further data collected should include risk factors for wound healing, such as diabetes and the use of steroids. In addition, procedural factors, such as the size of the excision, the co-administration of antibiotics or adrenaline and the timing of suture removal and follow-up, should be recorded.
The use of a standardised wound assessment tool would reduce interclinician variability and improve the reliability of complication data.28 Furthermore, collecting pain scores during and immediately after the procedure would provide evidence about the effectiveness of anaesthesia. Research should be performed collaboratively with GPs and external researchers, because GP involvement is essential to assist clinic and patient recruitment and ensure applicability in primary care.29,30 Clinic nurses and medical students can be used for patient recruitment and data collection, as well as to reduce costs.29
Conclusion
This study identified a previously unreported positive association between lignocaine dose and volume and the rates of complications in skin cancer lesion excisions. Furthermore, there was a significant reduction in both the dose and volume of lignocaine administered after a simple educational intervention. However, the study methodology and confounders make it difficult to attribute the effects of dose and volume on eventual complication rates. Given this, further research is warranted to evaluate these hypotheses that could take the form of a multiclinic prospective randomised control trial.