Acute otitis externa (AOE), a common inflammatory condition of the outer ear, frequently occurs in tropical climates such as North Queensland.1–3 Often seen by general practitioners (GPs), patients exhibit signs and symptoms of external auditory canal (EAC) inflammation, frequently preceded by factors that increase EAC vulnerability.1,4,5 Treatment with topical antibiotics and ear cleaning typically lead to significant improvement within 72 hours.4–6 However, complex or untreated cases, especially among diabetics, might lead to severe complications like necrotising otitis externa (NOE).7–9 This article aims to present key aspects of AOE and outline an effective management approach aligned with the clinical practice guideline by the American Academy of Otolaryngology Head and Neck Surgery (AAO‑HNS).4
Aim
The aim of this study is as follows:
- Outline key aspects of AOE and distinguish features to its differential diagnoses.
- Identify common risk factors and specific populations at increased risk of developing AOE and its complications.
- Propose a streamlined management approach for AOE for GPs.
- Provide key features that necessitate referral to otorhinolaryngology.
Approach
Anatomy
The outer ear comprises the pinna (auricle) and the EAC, an S-shaped passage from the pinna to the tympanic membrane (TM).10 The EAC consists of a cartilaginous outer third and a bony inner two-thirds. The cartilaginous part has hair-bearing skin and small perforations (fissures of Santorini), whereas the bony section, part of the temporal bone, is lined with thin stratified squamous epithelium.8–10 The exact shape of the EAC differs between individuals, and canals that are tortuous, narrow or collapsing, or bony growths like exostoses, translate to a greater risk of otitis externa.
History
Establishing a clear timeline is crucial to identify predisposing factors to EAC injury and differentiating AOE from other conditions.4,6 Direct trauma to the EAC and water exposure are the most common causes of AOE.4–6 Water exposure predisposes the EAC to bacterial buildup, whereas devices like hearing aids hinder ventilation and cerumen clearance.2,4,5 Use of cotton buds in the ear canal causes local injury that allows pathogens to bypass the skin barrier.4–6 Diabetes is a significant risk factor associated with microangiopathy along the canal, reduced immune response and impaired wound healing, increasing susceptibility to AOE and NOE.8,11 Immunosuppressed individuals have ineffective immune response, whereas dermatological conditions weaken the EAC lining.4–6,8 Radiotherapy leads to compromised skin integrity, reduced blood supply to the soft tissues and bone, as well as potential narrowing of the EAC.4,5 Table 1 outlines AOE-associated risk factors.
| Table 1. Risk factors for acute otitis externa1,2,4–6,14 |
| Risk factors |
Examples |
| Water/moisture exposure |
- Swimming, spa/hot tubs, bath/shower, sweating, humidity/climate (wet season, summer)
|
| Local injury |
- Instrumentation (eg cotton buds), foreign bodies, ear syringing/cleaning
|
| Canal obstruction |
- Hearing aids, earplugs, ear protection, earphones/headphones
- Foreign bodies, canal stenosis, canal exostoses, excess hair in the canal
|
| Comorbidities |
- Diabetes mellitus, immunosuppression, chronic suppurative otitis media, cerumen impaction, previous radiotherapy
- Dermatological (atopic/contact dermatitis, psoriasis, eczema, seborrhoeic dermatitis, keratosis obturans)
|
Aboriginal and Torres Strait Islander population
Indigenous Australians have a lower life expectancy and higher risk of otorhinolaryngological conditions.12 Diabetes is the second leading cause of mortality, showing a prevalence 2.8-fold higher than in non-Indigenous populations.13 A retrospective analysis in the Northern Territory revealed that among nine patients with NOE, six were Indigenous, all of whom were diabetic and aged around 16 years younger than their non-Indigenous counterparts.11 Therefore, clinical vigilance is advised when assessing Indigenous patients with acute otalgia due to a higher likelihood of NOE.
Examination
The pinna and tragus might show erythema, oedema and acute tenderness, with periauricular cellulitis and postauricular lymphadenopathy.2,4,6 Otalgia can be elicited by tragal pumping and pinna retraction. During otoscopy, carefully advancing the speculum allows further evaluation of canal inflammation, which is often painful and necessitating analgesia beforehand.4 The EAC typically appears erythematous, oedematous and moist.2,4,6 Otorrhoea or debris in the EAC can provide diagnostic clues regarding the causative pathogen. Bacterial cases are non-specific, whereas fungal causes, like Aspergillus species (white hyphae with black spores) and Candida species (off-white sebaceous-like debris) can be differentiated.2,4 EAC might be swabbed if empirical treatment fails and the exact pathogen needs to be identified to guide antibiotic therapy. The integrity of the TM is imperative in choosing appropriate topical antibiotics, as AOE can cause secondary perforation to allow communication into the middle ear.4
Diagnosis and differentials
AOE usually involves identifiable risk factors and three key features, as per the 2014 AAO-HNS guideline.4 These include symptoms (otalgia, pruritus and aural fullness) and signs (tenderness, oedema and erythema) of EAC inflammation with rapid onset within 48 hours.4 Acute tragal tenderness is widely accepted as a pathognomonic sign. Predominantly bacterial, AOE is commonly caused by Pseudomonas aeruginosa or Staphylococcus aureus.1,2,4–6 Fungal causes (otomycosis), like Aspergillus and Candida species, often manifest as co-pathogens, in immunocompromised patients, or after prolonged use of topical antibacterial drops.2,4,6 Both bacterial and fungal AOE have overlapping features of otalgia, otorrhoea, pruritus and erythema. Thus, otoscopy, swabs and contextual cues from history are necessary to confirm fungal AOE. Table 2 outlines clinical features that are useful in distinguishing AOE from its differential diagnoses.
| Table 2. Distinguishing features of differential diagnoses of AOE4–6,14,19 |
| Differential diagnoses |
Distinguishing features |
| Otological |
| AOM/CSOM |
- Presence of middle ear effusion, which appears as dull or yellow hue behind the TM, and the TM might be bulging towards the canal
- If the TM is intact, the EAC and pinna inflammation should be absent. The TM will be immobile on pneumatic otoscopy
- If the TM is perforated, EAC might be inflamed secondary to otorrhoea
|
| Mastoiditis |
- Typically, a complication of AOM with features of AOM, as described above
- The posterior wall of the EAC might be bulging on otoscopy
- Signs of inflammation (tenderness, erythema, fluctuance) will be present over the mastoid cavity, and the pinna might be protruded anteriorly
|
| Furunculosis |
- Presence of a fluctuant, erythematous nodule in the distal EAC (‘pimple-like’) as a focal source of infection
|
| Perichondritis |
- Significant pinna inflammation with lobule sparing (as it does not contain any cartilage) and the EAC is not involved
|
| Neoplasm/abnormal growth |
- Examples: cholesteatoma, SCC, BCC, melanoma
- Can be indistinguishable from AOE, and should be considered if patients are unresponsive to AOE treatment
- Might be constitutional symptoms and focal neurological signs (eg facial nerve palsy)
|
| Inflammatory |
| Contact dermatitis |
- Might be secondary to materials (eg metals, plastics), chemicals (eg soap) or medications (eg topical antibiotics), which is often identifiable in history
- Pruritus is the predominant symptom, with variable degrees of otalgia
- Symptoms are reversible with the removal of the offending agent
|
| Ramsay Hunt syndrome (herpes zoster oticus) |
- Complication of shingles due to the varicella-zoster virus affecting the facial nerves
- Typically exhibits erythematous vesicles in the EAC, the pinna and the tongue, with neuropathic otalgia and facial nerve paresis (facial droop, loss of taste)
|
| Dermatological |
| Eczema (atopic dermatitis) |
- Pruritus is the predominant symptom, and the EAC can appear erythematous with crusting and exudation, without significant oedema
- There might also be fissuring around the infra-auricular and retroauricular folds
|
| Seborrhoeic dermatitis |
- Fungal skin infection by Malassezia species, exhibiting scaly erythematous plaques around the scalp, pinna, face and trunk
- EAC inflammation is absent
|
| Psoriasis |
- Erythematous and scaly plaques can involve the EAC and the pinna, which might be bilateral with sharply defined margins
- Pruritus is the main symptom, and otalgia might be absent
|
| Others |
| TMJ disorder |
- Absence of EAC and pinna inflammation
- History of TMJ injury (eg trauma, bruxism, dental procedure/malocclusion) or repetitive jaw motions (eg gum chewing)
- Masticator muscle pain on palpation, TMJ crepitus
|
| AOE, acute otitis externa; AOM, acute otitis media; EAC, external auditory canal; SCC, squamous cell carcinoma; TM, tympanic membrane; TMJ, temporomandibular joint. |
Management
Antibiotic choice
Topical antibacterial ear drops, either quinolone based or non-quinolone based, are the first-line treatment for AOE and are empirically commenced without the need for swab confirmation because a significant proportion of cases are bacterial in origin.4 Generally, 65–90% of patients experience clinical improvement within 7–10 days, regardless of the specific topical formulation used.4,5
Non-quinolone-based agents are cost-effective and offer comparable efficacy but possess potential ototoxic effects in the middle ear.2,4 In contrast, quinolone-based agents are not ototoxic and are advised for AOE with TM perforation or when TM integrity cannot be assessed, but they tend to be more expensive for patients.4 Although non-quinolone-based agents are advised for use in AOE cases with intact TM given their lower costs, the dilemma in general practice is that TM integrity is difficult to adequately ascertain due to pain, swelling, debris and lack of access to microscopes or microsuction. Quinolone-based agents are thus advised in these circumstances to ensure timely treatment is given without potentially causing ototoxicity. A list of topical agents available in Australia is provided in Table 3.3,14–16
| Table 3. Topical ear drops available in Australia3,14–16,19,20 |
| Name (Brand name, manufacturer) |
Indication |
DosingA |
Average price ($)B |
Comment |
| Quinolone based |
| Ciprofloxacin 0.3% (Ciloxan, Novartis Pharmaceuticals Australia Pty Limited, Macquarie Park, NSW) |
AOE ± TM perforation |
5 drops into the affected ear, twice a day until a few days after symptoms have cleared |
31 |
- Treatment should not exceed 2 weeks
|
| Ciprofloxacin 0.2% + hydrocortisone 1% (Ciproxin HC, Novartis Pharmaceuticals Australia Pty Limited, Macquarie Park, NSW) |
AOE ± TM perforation |
3 drops into the affected ear, twice a day until a few days after symptoms have cleared |
47 |
- Treatment should not exceed 2 weeks
|
| Non-quinolone based |
| Framycetin 0.5% (Soframycin, Sanofi-Aventis Australia Pty Ltd, Macquarie Park, NSW) |
AOE |
3 drops into the affected ear, 3 times a day until a few days after symptoms have cleared |
12 |
- Not suitable for TM perforation
- Treatment should not exceed 2 weeks
|
| Framycetin 0.5% + gramicidin 0.005% + dexamethasone 0.05% (Sofradex, Sanofi-Aventis Australia Pty Ltd, Macquarie Park, NSW) |
AOE |
3 drops into the affected ear, 3–4 times a day until a few days after symptoms have cleared |
11 |
- Not suitable for TM perforation
- Treatment should not exceed 2 weeks
|
| Neomycin 0.25% + gramicidin 0.025% + triamcinolone 0.1% (Kenacomb Otic, Otocomb Otic, Aspen Pharmacare Australia Pty Ltd, St Leonards, NSW) |
AOE, otomycosis |
3 drops into the affected ear, 2 or 3 times a day until a few days after symptoms have cleared |
11 |
- Not suitable for TM perforation
- Treatment should not exceed 2 weeks
|
| Clioquinol 1% + flumetasone 0.02% (Locacorten-Vioform, Amdipharm Mercury (Australia) Pty Ltd, North Sydney, NSW) |
Otomycosis |
3 drops into the affected ear, twice a day until a few days after symptoms have cleared |
23 |
- Not suitable for TM perforation
- Treatment should not exceed 2 weeks
|
| Clotrimazole 1% (Canesten, Bayer Australia Limited, Gordon, NSW) |
Otomycosis |
3 drops into the affected ear, twice a day until a few days after symptoms have cleared |
11 |
- Not suitable for TM perforation
- Treatment should not exceed 2 weeks
|
| Non-antibiotic |
| Acetic acid 1.73% + isopropyl alcohol 63.4% (Aqua Ear, Haleon Australia Pty Ltd, Parramatta, NSW) |
Prevention of AOE |
4–6 drops into each ear post water exposure |
11 |
- Not suitable for TM perforation
- Stinging sensation on instillation common
|
| Docusate Sodium 0.5% (Waxsol, Viatris Pty Ltd, Millers Point, NSW) |
Cerumen clearance |
Fill the ear canal on 2 consecutive nights |
13 |
- Not suitable for TM perforation
|
| Carbamide peroxide (Ear Clear, Key Pharmaceuticals Pty Ltd, North Ryde BC, NSW) |
Cerumen clearance |
5–10 drops twice daily for up to 4 days |
13 |
- Not suitable for TM perforation
|
AFrom Australian Medicines Handbook.
BCalculated from prices listed online on Chemist Warehouse, Your Discount Chemist and Chempro Chemist, as of December 2023.
AOE, acute otitis externa; TM, tympanic membrane. |
Systemic antibiotics are avoided for uncomplicated AOE, as topical agents already provide concentrations 100- to 1000-fold higher than their systemic counterparts.4 They also do not expedite clinical resolution or pain relief compared to topical treatment.4 However, they are warranted in cases extending beyond the EAC (eg periauricular cellulitis) or involving high-risk populations such as diabetics and immunocompromised patients.4 The local infectious disease team should be consulted about appropriate systemic antibiotic treatment due to the limited anti-pseudomonal agents available and the rising antibiotic resistance.4,8
Drug delivery
Ear drops are administered with the affected ear facing up, completely filling the EAC, and this is ideally done by another person.3,4 The patient should maintain this position for five minutes for sufficient penetration, with tragal pumping to displace the air if required.3
Microsuction is a form of ear toilet and is strongly advisable where available to clear otorrhoea and debris in the EAC.7 Gentle dabbing with a tissue spear is an alternative, but twisting should be avoided due to shearing injury and pain. Multiple sessions of ear toilet are often required in conjunction to antibiotic therapy to ensure timely resolution. Irrigation is strongly discouraged, especially in suspected acute otitis media (AOM), and in diabetic and immunocompromised populations due to increased risk of NOE.4,17 Wick insertion might be necessary if EAC oedema precludes ear drop penetration.1,4,6 Pope Wicks are expandable rectangular sponges designed for EAC insertion; 15 mm is recommended, as the average EAC length is 25 mm.10 Agents such as Kenacomb Otic might be applied pre-insertion, but this is not necessary as the primary function of the wick is to allow topical antibiotic solution to impregnate and expand the wick for better canal contact and penetration. The lateral end of the Pope Wick must be visible to ensure proper reach by the topical agent. As improvement is expected within 72 hours, the wick should be removed at this point and the EAC should be reassessed.4
Analgesia
Paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs) are effective pain treatment, especially in the acute phase.4 Regular dosing is recommended over ‘as needed’ dosing for sustained analgesia.4 Opioids are reserved for severe pain or before procedures, but they are rarely necessary because most patients improve within 72 hours.4 Topical analgesics are discouraged due to limited efficacy and the potential to interfere with topical agents in the canal.4 The steroid contained in the combination topical antibiotic formulation helps alleviate pain by reducing local inflammation and should be taken into account when prescribing.
Refractory cases
Patients without clinical improvement within 72 hours of treatment should be reassessed, to ensure patient compliance and that the EAC is adequately receiving topical treatment.4 Patients might require repeat microsuction or wick insertion, and an ear swab can be performed if a non-pseudomonal cause (eg other bacteria, fungal) is suspected. Patients with persistent or worsening otologic symptoms, or those developing cellulitic or neurological signs, should be investigated for the possibility of NOE.
Prevention
Ear plugs prevent water retention in the EAC during water-based activities, and petroleum jelly applied around the ear plugs can enhance the seal.3,4 A hair dryer on the lowest heat setting or applying acetic acid/ethanol ear drops can dry the EAC.3,4 Individuals who use hearing aids or similar devices should take frequent breaks for ventilation and clean the earpieces regularly. Cerumenolytics prevent cerumen impaction, whereas instrumentation (eg cotton buds) should be avoided.3,4 Effective management of predisposing conditions like diabetes mellitus is crucial and might necessitate consultation with relevant specialists.3,4,7
Indication for referral
Referral to otorhinolaryngology is recommended if the condition worsens despite appropriate treatment or if the condition persists beyond two weeks.3,14 If significant debris or otorrhoea impedes drug delivery and clearance is not feasible in general practice, microsuction might be necessary and warrants referral. Likewise, if severe EAC oedema precludes wick insertion, referral is advisable.
Patients showing focal neurological signs (eg facial nerve palsy) suspicious of NOE should have imaging and an urgent otorhinolaryngology consultation through the emergency department (ED).3,4 Cases with systemic features indicating infection beyond the EAC (eg periauricular cellulitis) also warrant prompt evaluation and potential hospital admission.3,4 A flowchart for AOE management is shown in Figure 1.
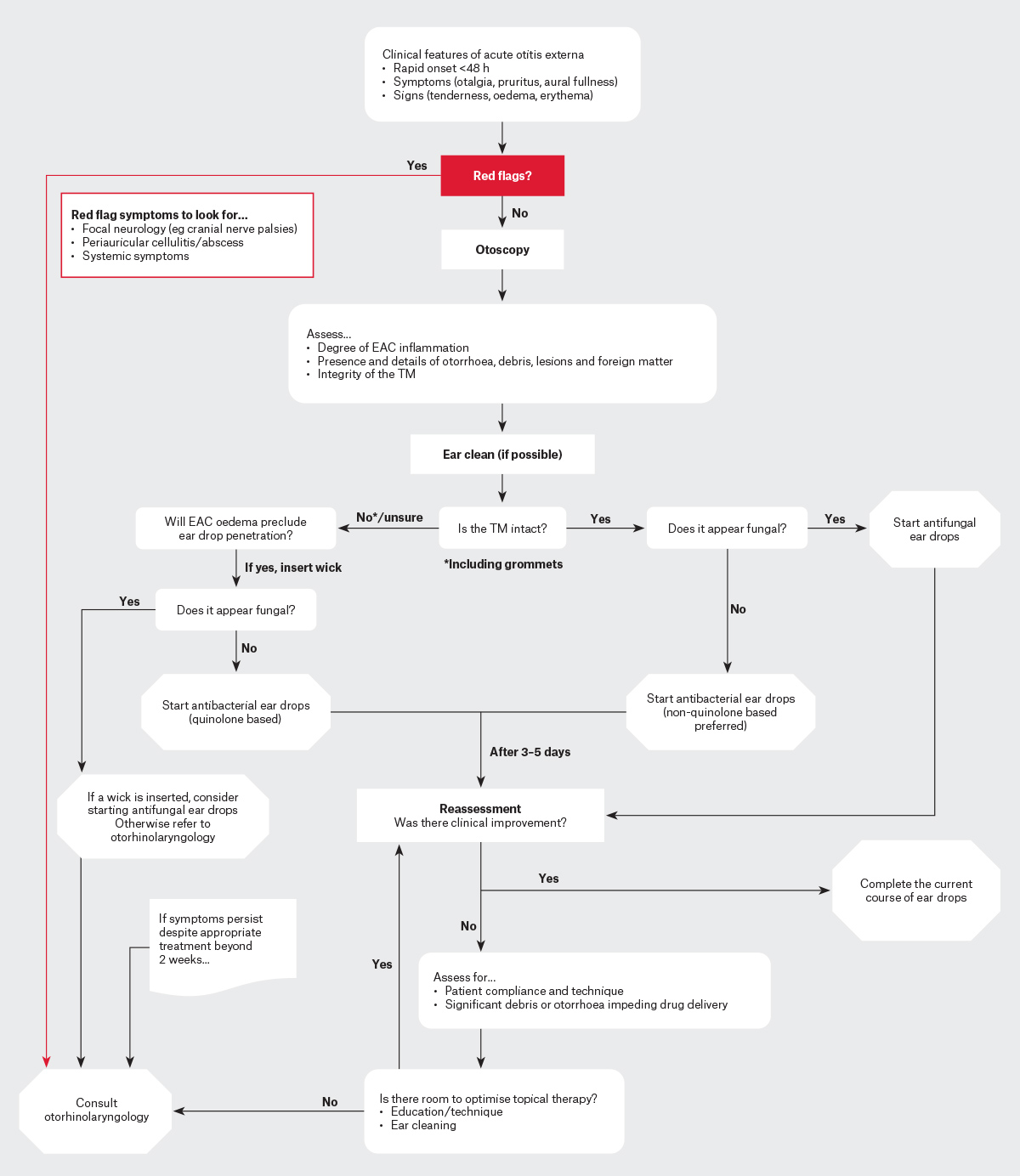
Figure 1. Flow chart for managing acute otitis externa in the community.
EAC, external auditory canal; TM, tympanic membrane.
Complications
Necrotising otitis externa is a key complication of AOE, commonly observed in diabetic and immunocompromised populations.7,8 It was initially termed malignant otitis externa due to its aggressive spread and high mortality of up to 67%.9,18 However, the nomenclature has shifted to NOE to reflect its infectious aetiology, and the advent of anti-pseudomonal antibiotics has reduced patient mortality to 2–15%.8,18 It is osteomyelitis of the temporal bone and skull base, which can potentially progress to skull base osteomyelitis (SBO), multiple cranial nerve palsies, meningitis and brain abscesses.7,8,18
Necrotising otitis externa develops when AOE spreads medially via the fissures of Santorini, affecting the bony two-thirds of the EAC, the stylomastoid foramen (CN VII), the mastoid tip and the jugular foramen (CN IX-XI).8,17 This progression leads to cortical bone erosion and granulation tissue formation, often visible in the EAC.7–9 Management generally necessitates hospital admission under otorhinolaryngology with infectious disease consultation, requiring extended targeted antibiotic treatment lasting at least four to six weeks.7–9
AOE might cause cellulitis and abscess formation in periauricular soft tissues, requiring incision and drainage.9,17 Perichondritis of the pinna can also occur, resembling features of AOE, but spares the earlobe because of the absence of cartilage.
Conclusion
In conclusion, AOE is a common condition, particularly in warm, humid climates, and is predominantly managed by GPs. Early identification of symptoms and risk factors, with timely intervention involving topical antibiotics and ear cleaning, are key to rapid clinical improvement. Complications, especially among diabetics, might arise, highlighting the importance of vigilance and proactive management in general practice. This article aims to equip GPs with essential insights and management strategies in alignment with the AAO-HNS clinical practice guideline, emphasising the importance of primary care in preventing potential complications and ensuring optimal patient care.
Key points
- AOE is a common condition seen in general practice and is predominantly bacterial in origin.
- A clear timeline is crucial to identify risk factors and differentiate AOE from other conditions.
- Topical antibiotics is recommended as gold standard treatment for AOE.
- NOE is a serious complication of AOE that requires otorhinolaryngology referral.
- Indigenous Australian, diabetic and immunocompromised individuals are at higher risk of AOE and its complications.