Chronic rhinosinusitis (CRS) is a common medical condition managed by Australian general practitioners (GPs) and accounts for up to 1.4 in every 100 consults.1 It affects up to 10% of the Australian population and has a significant effect on quality of life.2 The treatment paradigm of nasal saline irrigation, intranasal and oral corticosteroids, and endoscopic sinus surgery is well established.3 Despite compliance with medical therapy and appropriate surgery, some patients are troubled by persistent disease. There is now emerging evidence for the use of biologic agents in the management of CRS with nasal polyposis (CRSwNP).4 Mepolizumab, an anti-interleukin 5 monoclonal antibody, was added to the Pharmaceutical Benefits Scheme (PBS) in April 2023. As these agents become more commonplace, it is important for GPs to be aware of their use, indications and potential adverse events.
Aim
The aim of this article is to provide an update of the management of CRS with and without nasal polyposis for Australian GPs, including the role of biologic agents.
Background
CRS is a complex condition that results in inflammation affecting the mucosal surface of the nose and sinuses. The exact mechanism is not fully understood; however, it is thought that this inflammation is the result of both immune system and environmental factors, with many different inflammatory mediators involved. A close relationship between CRS (in particular, CRSwNP) and lower airway disease (asthma) has been observed, with some overlap in pathogenesis that results in a higher proportion of CRS sufferers also having asthma than the general population (and vice versa).5,6
Diagnosis
CRS manifests as four cardinal symptoms:
- nasal obstruction
- nasal discharge (anterior or posterior nasal drip)
- facial pressure/pain
- reduction or loss of smell.
To make a diagnosis of CRS, two of these symptoms must be present, including at least one of nasal obstruction or nasal discharge.3 Additionally, these symptoms should be present for more than 12 weeks. The examination findings suggestive of CRS are summarised in Box 1.
| Box 1. Examination findings in chronic rhinosinusitis |
- Mucopurulent rhinorrhoea
- Nasal polyps
- Oedematous nasal mucosa
|
Imaging
Treatment for CRS if often commenced prior to cross-sectional imaging being performed. However, computed tomography (CT) is useful to confirm the diagnosis or in complicated or refractory cases.
CT findings in CRS include:
- mucosal thickening
- partial or complete opacification of one or more sinuses
- hyperostosis of one or more sinuses
- obstruction of the ostiomeatal complex and extension of opacification into the nasal cavity
- presence of nasal polyposis.
In addition to diagnosis, CT plays a crucial role in preoperative planning and is required prior to surgical intervention.
Red flag symptoms
There are several symptoms that, if present, require urgent cross-sectional imaging and prompt referral to an otolaryngologist for further investigation.3 These include:
- orbital symptoms – diplopia, ophthalmoplegia and peri-orbital oedema
- unilateral symptoms
- bloody rhinorrhoea
- abnormal neurology
- severe headache
- signs of meningitis or sepsis.
Management
CRS is best treated with a multimodal approach (Figure 1). Many patients present to primary care clinics looking for a quick and permanent solution to their symptoms of CRS, but the reality is, the disease process requires ongoing management. Thus, patient education and adherence to medication regimes is of the utmost importance.
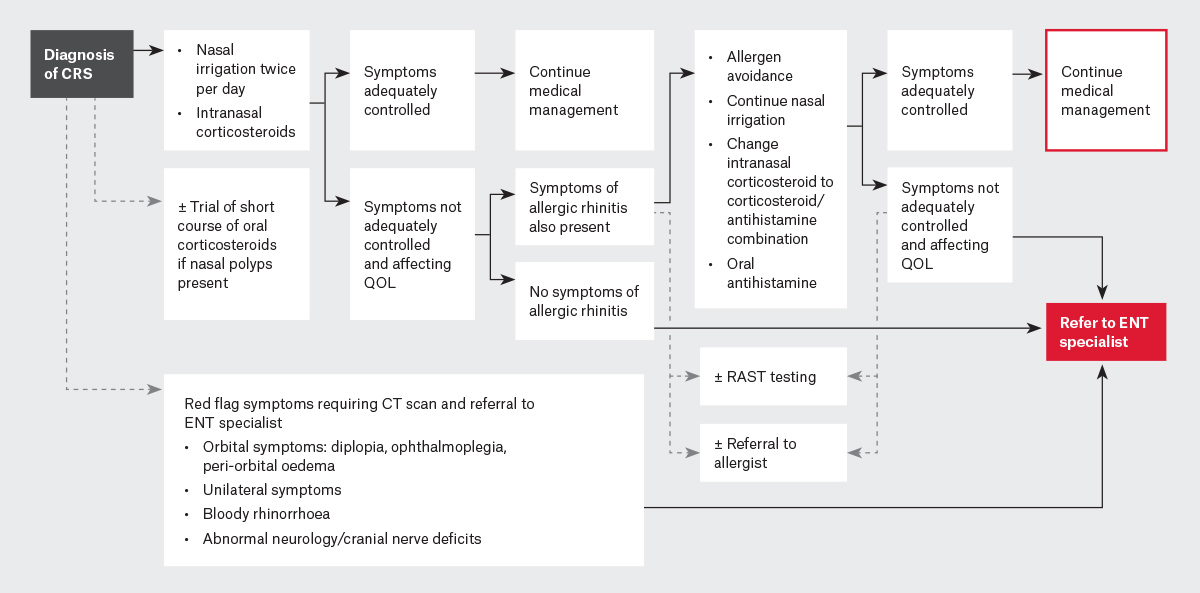
Figure 1. Flowchart for the management of chronic rhinosinusitis.
CRS, chronic rhinosinusitis; CT, computed tomography; ENT, ear, nose and throat; QOL, quality of life; RAST, radioallergosorbent test.
Nasal irrigation with high volume saline irrigation solutions should be used at least twice per day. This can improve sinonasal mucosal function in several ways, including mechanical lavage of mucus, optimisation of mucociliary function, and removal of allergens, antigens and inflammatory molecules.3 Additionally, intranasal corticosteroids such as budesonide or mometasone should be used daily following nasal irrigation, and patients should be counselled that they will not notice instant relief of symptoms, as continued use will allow for the treatment to take effect.
In 2014, nasal sprays that contain both corticosteroids and antihistamines became available in Australia.7 These medications can treat CRS through the actions of corticosteroids, but they do also have action against allergic rhinitis through the actions of both corticosteroids and antihistamines.8 Allergic rhinitis can overlap with CRS and cause symptoms such as nasal congestion, rhinorrhoea, sneezing and itch. For these patients, oral antihistamine medications might also provide some benefit. The diagnosis of allergic rhinitis can often be elicited on history but can be aided by radioallergosorbent (RAST) blood testing or skin prick testing. Although antihistamine medications can help, other lifestyle strategies such as avoidance of the allergen can be useful. If these fail, then desensitisation immunotherapy can be considered.
Short courses of oral corticosteroids do play a role in the management of patients with CRS with polyps. These have been shown to reduce the size of intranasal polyps but should only be used for limited periods and in conjunction with nasal irrigation and nasal sprays.9 The course length and dose depend on a patient’s past medical history and the prescriber’s individual preference.
If a patient is still affected by the symptoms of CRS following compliance with a trial of maximal medical therapy, then a referral to an otorhinolaryngologist should be made for further evaluation and consideration of alternative medical therapy or surgical intervention (most commonly, functional endoscopic sinus surgery [FESS]) for medically refractory patients. The aim of sinus surgery is to improve mucociliary drainage pathways and allow topical therapies to provide greater benefit to the patient. By removing polyps (if present), widening sinonasal ostia and restoring aeration of sinuses, mucus can drain more freely. Additionally, surgery allows more efficient delivery of topical corticosteroids and nasal saline irrigation to the sinonasal mucosa.
Other procedures, such as septoplasty or inferior turbinate reduction, can be performed in conjunction with FESS to improve the nasal airway if anatomical obstruction of airflow is occurring.
It is important to counsel patients that surgery does not address the pathophysiology of CRS; thus, nasal irrigation and topical corticosteroids must still be used postoperatively.
Unfortunately, 14–24% of patients undergo revision sinus surgery at some stage.10 Additionally, this surgery is likely to carry a higher complication rate than the primary surgery.11
Management with biologic agents
Biologic agents have been used for obstructive airway disease and off-label for CRS for several years. In April 2023, one of these agents, mepolizumab, was added to the PBS for the treatment of CRS with polyps.
Mepolizumab is a monoclonal antibody that targets interleukin 5. It is given via monthly subcutaneous injection and has been shown to reduce polyp size and decrease nasal obstruction scores in patient with CRSwNP.12 It is generally well tolerated and is required to be continued in an ongoing manner for the treatment effect to be maintained.
Mepolizumab has been found to result in a 47% reduction in nasal congestion (measured by the nasal congestion score [NCS]) and a 45% improvement in the Sinonasal Outcome Test 22 (SNOT-22) score (a quality-of-life outcome measure for CRS) after one year.4 It only resulted in a 17% reduction in nasal polyps (measured by the nasal polyposis score [NPS]).4
To be eligible for mepolizumab on the PBS, patients must have a confirmed diagnosis of CRSwNP, with a blood eosinophil count of ≥300 cells/µL, and have persistent disease despite previous surgery (or be unsuitable for surgical management). They must have moderate–severe disease as measured by endoscopic polyp score >5, nasal obstruction visual analogue score >5 and symptom visual analogue score >7. They must also have demonstrated compliance with medical therapy.
Although only one biologic agent is currently PBS listed for use in CRS, multiple biologic agents have been used and tested. These have the potential to be used more in Australia in the future.
For example, another biologic agent, dupilumab, which targets the interleukin-4 receptor, resulted in a 36% reduction in NPS and a 58% reduction in the SNOT-22 score at one year.4 Omalizumab, which targets free IgE, resulted in a 21% reduction in NPS and a 48% reduction in NCS at one year.4
Notably, several biologic agents are PBS listed in Australia for patients with severe asthma, including mepolizumab, omalizumab, dupilumab and benralizumab. As discussed earlier, there is significant overlap between asthma and CRS, with reports of 35–45% of patients with severe asthma suffering from CRS as well.13,14 Thus, biologic agents in these patients have the potential to provide benefit for both asthma and CRS symptoms.
There are several potential side effects of mepolizumab that GPs should be aware of as it becomes more commonly prescribed in Australia. Mild side effects include irritation at the injection site, headache and joint pain. Reactions that have the potential to be more serious include varying degrees of allergic reaction (including anaphylaxis) and an association with herpes zoster (shingles), which occurs in < 0.1% of patients.15
Conclusion
CRS is a common and potentially difficult medical condition to manage. It requires persistence from both patients and medical providers to achieve adequate control of symptoms. The addition of a biologic agent (and potentially more to come in the future) to the PBS for treatment of CRSwNP provides another option for those with persistent disease despite appropriate medical and surgical management.
Key points
- CRS is a common medical condition that, if poorly treated, can significantly affect a patient’s quality of life.
- A multimodal management approach, including nasal irrigation and intranasal corticosteroids, is required.
- Functional endoscopic sinus surgery can be offered for patients who have symptoms that are inadequately controlled with non-operative management.
- A biologic agent, mepolizumab, is now available in Australia to use for refractory cases of CRS with polyposis.
- Biologic agents are effective at improving symptoms of CRS and are generally well tolerated.