Anal fissure (AF) is characterised by a superficial tear in the anoderm distal to the dentate line.1,2 It is one of the most common anorectal problems presenting in healthcare settings, second only to haemorrhoids, and affects both genders equally.3 Estimates of AF frequency in the general population are 10–15%, although the actual number might be higher due to patients not presenting to healthcare providers.1,3 Despite this prevalence, a 2019 study of colorectal surgeons in Australia and New Zealand showed genuine clinical uncertainty in approximately two-thirds of clinical management questions.4 General practitioners and emergency medicine specialists are often the first points of contact for AF presentation and acute management. However, if medical treatments fail, it is imperative to refer patients on to a general or colorectal surgeon for further evaluation and treatment.5
The aim of this article is to provide a comprehensive summary of the pathophysiology, clinical presentation and management of AF under current guidelines. Patients with obstructed defaecation requiring anorectal manometry testing and other surgical treatments are not discussed.
Clinical presentation and assessment
Patients with AF will present with severe tearing pain with defaecation, often accompanied by spots of bright red blood.2 This pain can persist for one to two hours or more afterwards, causing many patients to fear defaecation and worsen symptoms of constipation as a result. AF is observed in patients of any age, although the differential diagnosis differs.1,3
Definitive diagnosis is often achieved with visual inspection. The patient might lie in the lateral decubitus position or prone over a table flexed at the hips. Once in position, gentle parting of the buttocks might reveal the fissure.2 Readers might recall the classic Hamilton Bailey description when looking for a fissure of a canoe-shaped ulcer.6
Acute fissures present for six weeks or less are seen as a fresh laceration on the sphincter upon examination (Figure 1A,C). Acute AF often heals spontaneously within 4–6 weeks or with medical management, although approximately 40% will progress to chronic fissures.1,5 Chronic fissures often have raised edges and exposed internal anal sphincter (IAS). Distal sentinel tags and hypertrophied anal papilla at the internal apex might also be present (Figure 1B,D).1
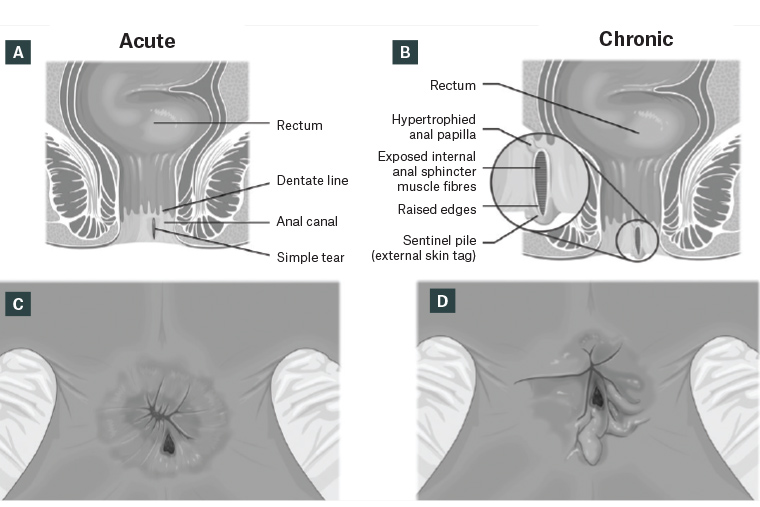
Figure 1. Coronal view of anal canal with acute anal fissure (A) and chronic anal fissure and sentinel tag (B). Lithotomy perineal view of acute anal fissure (C) and chronic anal fissure with sentinel tag (D).
If a fissure is not apparent, gently pressing on the anterior or posterior anal sphincter might produce the characteristic pain.5 Digital exam is contraindicated with suspected AF due to this pain. If the diagnosis remains unclear or if there is a co-occurring fistula, examination under anaesthesia using anoscopy, endoscopy or biopsy might be performed.2
Pathophysiology
The anal canal is approximately 3–5-cm long, divided into two parts by the dentate line.1,7 The aetiology of the characteristic superficial tear of AF in the anoderm is unclear, although many theories have been posited and correlations found with other conditions.1 Data supports the idea that AF occurs due to localised ischaemia augmented by IAS hypertonicity.8 This theory corresponds to the high prevalence of fissures in the posterior midline (90% of all presenting AF cases), an area that receives less than half of perfusion compared to the rest of the anal canal.5
Primary AF might be idiopathic but is often correlated with local trauma, such as hard stools, prolonged diarrhoea, vaginal delivery or anal penetration. An acute injury can trigger spasms of the IAS, causing IAS resting hypertonicity. The resulting pressure on blood vessels supplying the area decreases perfusion, causing ischaemia and hindering healing of the fissure.2 As a result, most treatments focus on reducing IAS resting tone. However, fissures anterior to the midline are usually related to occult external sphincter injury and often lack IAS hypertonicity. These fissures require alternative treatment methods.1
Secondary AF, comprising less than 1% of AF presentations, is often characterised by multiple fissures or fissures off midline.1 These fissures are associated with a variety of histories, including patients with previous anal surgeries, inflammatory bowel disease (especially Crohn’s disease), granulomatous diseases (eg tuberculosis and sarcoidosis) and gastrointestinal malignancies (eg colon cancer).2 Sexually transmitted infections might also present with AF in addition to discharge, perianal ulcers or fever. If a secondary AF is suspected, surgical therapies should be postponed pending further investigation and multidisciplinary involvement.2
Management
Non-surgical management
Management should aim to reduce constipation, relieve pain and heal the fissure. Risk of complications, including sepsis, fistula formation and chronic fissure development, should be minimised.
Conservative therapy
The American Society of Colon and Rectal Surgeons (ASCRS) guidelines suggest a combination of increased fibre intake, sitz baths and, if necessary, stool softeners as the first-line therapy for acute AF or chronic AF in patients who elect or are unable to have surgery.4 These methods alone or in addition to pharmaceutical treatments are safe and have few side effects.1 Conservative therapy has shown a healing rate of 87% with 15 g/day of fibre supplementation. This therapy reduced recurrence at 16% versus 68% with placebo.3
Topical nitrates
Glyceryl nitrate (GTN) ointment is often used for acute AF treatment. GTN undergoes cellular metabolism to release nitric oxide, which relaxes the IAS by acting as an inhibitory neurotransmitter.1 A Cochrane review compared GTN to placebo and found GTN to be slightly but significantly better (49% vs 37%, P<0.004), although recurrence was widespread (>50%).1
A randomised control trial performed by Sahid et al found that co-administration of GTN and topical metronidazole decreased pain with defection during the early stages of treatment and significantly increased healing of AF by the end of week 6 (97.1% vs 85.2% with GTN alone, P<0.014).9
Significant side effects include headaches, light-headedness and local irritation. The severity of these side effects causes up to 20% of patients to discontinue therapy.1
Calcium channel blockers
Topical calcium channel blockers (CCBs) relax the IAS by blocking calcium influx to the cytoplasm of smooth muscle cells.1 ASCRS guidelines recommend CCB when GTN therapy has failed.4 The two most used CCBs are topical nifedipine (0.2–0.5% gel) and diltiazem (2% cream).2 CCBs have been shown to be as effective as GTN and are favoured for patients with poor reactions to GTN, as reported adverse effect rates are much lower, with only some experiencing mild headaches and anal pruritis.1,3 Reported healing rates vary from 65% to 96%.3
Botulinum toxin
Botulinum toxin A (BTA) is produced by Clostridium botulinum and acts as an inhibitory neurotransmitter by preventing acetylcholine release from presynaptic terminals. This causes relaxation of both the external and IAS for up to three months.1 ASCRS guidelines recommend BTA when topical therapy has failed.4 It is strongly recommended in patients at high risk of developing incontinence from surgery, including those with Crohn’s disease or a history of anal surgery.3 Adverse effects include short-term incontinence but at lower rates than surgical methods.7 A 10-year retrospective study by Brisinda et al found higher doses and injection at the level of the anterior commissure to have the best healing rates and lowest rates of recurrence.10
One prospective study showed comparable healing rates between GTN, diltiazem and BTA of nearly 55%. Additionally, diltiazem and BTA had lower reported adverse effects than GTN.4 Long-term recurrence might be up to 42%, depending on method.2
Surgical management
Surgical management of AF is typically recommended at least 6–8 weeks after attempting conservative treatment, although it might be the first-line treatment if the fissure is hyperalgesic or infected. The best results are seen with a combination of surgical and medical management.2,3
Lateral internal sphincterotomy
Lateral internal sphincterotomy (LIS) is most often used for managing chronic AF.8 This procedure consists of a partial incision to the IAS to relieve hypertonia.3
The significant adverse effect of this procedure is transient incontinence of flatus or stool in up to 17% of patients, with 1% experiencing faecal incontinence five years after the procedure.2,5 If LIS fails, the recommendation is a repeat sphincterotomy.4
Fissurectomy and advancement anoplasty
Fissurectomy consists of resecting the fibrous edges of the fissure, the sentinel skin tag and the hypertrophied anal papilla.3 Advancement anoplasty aims to cover the open wound with a skin or rectal mucous membrane flap.3 A Cochrane review favoured LIS in uncomplicated hypertonic AF cases but recommended combination fissurectomy/advancement anoplasty in AF patients with hypotonia when there is diagnostic doubt or if there is high risk for faecal incontinence with LIS.
ASCRS guidelines recommend adding anoplasty to BTA or LIS, as it increases the healing rate and decreases faecal incontinence at one year.4 Combination therapy of fissurectomy/diltiazem has also been recommended for chronic AF, as it has lower risk of perianal sepsis and incontinence with lower cost than BTA injections.11
Conclusion
AF is the second most common anorectal complaint. Diagnosis is often confirmed through history of severe pain with defaecation and visual inspection. Various non-surgical and surgical treatments are available based on the persistence and severity of the fissure.
Key points
- Posterior AF is more common than anterior AF and often accompanied by sphincter hypertonicity.
- Conservative therapy is the first line of treatment for managing acute AF.
- Non-surgical management should be attempted for at least 6–8 weeks.
- Chronic AF should be treated with a combination of medical and surgical management.
- Special considerations should be made for secondary AF and AF with hypotonia and in those at higher risk for incontinence.