We acknowledge individuals in the transgender community and people who are non-binary living with endometriosis who might not identify as women. Efforts have been made to use neutral language. Where the word ‘woman’ has been used, we include all people with a uterus.
Endometriosis is a chronic inflammatory condition defined as endometrial-like tissue proliferating outside the uterus. It is a common yet frequently under-recognised condition affecting one in nine Australian women and up to 50% of women experiencing infertility.1,2 The aetiology of endometriosis remains unclear, but genetic, hormonal and immunological factors play a role.3 Dysmenorrhoea, chronic pelvic pain, dyspareunia and infertility are some of the features of endometriosis and can cause significant morbidity. Moradi et al reported the average delay in the diagnosis of endometriosis in Australian women to be between seven and 12 years. Uncertainty and delays in diagnosis contribute to the psychological burden experienced by those with the condition.4 It is estimated that there are 23,400 endometriosis-related hospitalisations and there is a cost to the economy of $9.3 billion each year due to loss of quality of life and reduced productivity.5 A national action plan released by the Australian Government in 2017 acknowledged the significant physical and psychological burden that endometriosis poses to those affected by the disease and aims to improve awareness, clinical management and research into endometriosis.6 General practitioners are often the first point of contact in the presentation of pelvic pain and can provide valuable continuity of care in the multidisciplinary management of this complex condition.
Aim
This paper provides a summary of the three most recent evidence-based guidelines on endometriosis by the European Society of Human Reproduction and Embryology (ESHRE),7 the Royal Australian College of Obstetricians and Gynaecologists (RANZCOG)8 and the National Institute for Health and Care Excellence (NICE).9
Classification
The most recognised staging system (Table 1) is the revised American Society of Reproductive Medicine (rASRM) staging system.10 It does not predict the severity of symptoms or prognosis. Endometriosis is also commonly classified into three categories: superficial peritoneal disease, ovarian endometriosis and deep infiltrating endometriosis (DIE), defined as endometriotic lesions invading >5 mm beyond the surface of the peritoneum.11 Pelvic organs such as the bladder, bowel, pelvic wall and retroperitoneal structures are often involved. Rarely, endometriosis can occur outside the pelvis.
| Table 1. Revised American Society of Reproductive Medicine staging system10 |
| Endometriosis stage |
Description of lesions |
| Stage 1 |
- Minimal
- Few superficial implants (might be located on ovarian peritoneum)
|
| Stage 2 |
- Mild
- More and deeper implants (might be located on ovarian peritoneum)
|
| Stage 3 |
- Moderate
- Many deep implants
- Small cysts on one or both ovaries
- Presence of filmy adhesions
|
| Stage 4 |
- Severe
- Many deep implants
- Large cysts on one or both ovaries
- Many dense adhesions
|
| Endometriosis categories |
| Category 1: superficial peritoneal disease |
Endometriosis lesions adherent to the peritoneum |
| Category 2: ovarian endometriosis |
Involving cysts within the ovaries (chocolate cysts) |
| Category 3: deep infiltrating endometriosis |
Endometriotic lesions invading >5 mm beyond the surface of the peritoneum |
Risk factors and presentation
Risk factors that are recognised to be associated with a diagnosis of endometriosis include early menarche (≤12 years), shorter cycle length (≤26 days), heavy flow (excessive bleeding that affects quality of life), lean body size, nulliparity and presence of a first-degree relative with endometriosis.12 Women with endometriosis seem to consume fewer vegetables, fruits (particularly citrus fruits), dairy products, and foods rich in vitamin D and long-chain omega-3 fatty acids.13 However, there is no robust evidence demonstrating a significant association.7,13
Symptoms are highly variable and do not correlate well with disease stage.8 The three guidelines state that endometriosis should be considered in people (including those aged 17 years and under) presenting with one or more of the following clinical features: dysmenorrhoea, chronic pelvic pain, cyclical gastrointestinal symptoms (particularly dyschezia), cyclical urinary symptoms (particularly haematuria or dysuria), dyspareunia and infertility in association with one or more of the above.7–9 The ESHRE guideline also includes cyclical cough, haemoptysis and/or catamenial pneumothorax. Thoracic endometriosis is rare, but where present, co-existent pelvic endometriosis is reported in up to 55% of cases.14
Mechanisms of pain
Inflammatory mediators released by endometriotic lesions during menstruation include adhesion molecules, cytokines and nerve-sensitising growth factors that lead to anatomy-distorting adhesions and peripheral sensitisation of the pelvic viscera. In addition to dysmenorrhoea, this likely contributes to non-cyclical pelvic pain and dyspareunia.15 Somatic sensitisation of the pelvic floor muscles might cause stabbing pains and pelvic floor dysfunction. Over time, central sensitisation might develop and chronic pain is associated with psychological distress and fatigue.15
Diagnosis
Prompt diagnosis
The RANZCOG and NICE guidelines emphasise the importance of prompt diagnosis and treatment of people with suspected or confirmed endometriosis as delays in treatment might significantly affect quality of life and disease progression.8,9 Table 2 provides the RANZCOG recommendations on when to refer a patient to a gynaecologist. Young people (aged 17 years and under) with suspected or confirmed endometriosis should be referred to a paediatric and adolescent gynaecologist with an interest in endometriosis or to a gynaecologist who is comfortable treating adolescents with possible endometriosis. Box 1 provides a case discussion outlining a common clinical presentation, diagnosis and management of endometriosis.
| Table 2. When to refer |
| Consider referring people with suspected or confirmed endometriosis to a gynaecologist if: |
- Ultrasound or imaging are suggestive of a higher stage or deeply infiltrating disease (endometrioma, adenomyosis or disease invading other organs)
- The patient has severe, persistent or recurrent symptoms of endometriosis
- The patient has signs of endometriosis on examination
- Initial management is ineffective, not tolerated or contraindicated
|
| Box 1. Case discussion |
|
Georgie, aged 23 years, is a veterinarian science student who presents to her GP to discuss painful periods and pain during intercourse. She had her first period when she was 13 years old. She has always experienced pain, but it has worsened in the past 12 months. She is experiencing pelvic pain outside menses and 8/10 pain on days 1–3 of her cycle. This often results in her being bedbound and taking time off university and work. She has a regular 28-day cycle and she uses regular tampons, 6 hourly. She is in a stable relationship with her boyfriend of 2 years and has been experiencing pelvic cramping and stabbing pain during and for several hours after intercourse. She also describes regular bloating and pain with bowel motions during days 1–3 of her cycle. She has no urinary symptoms and no postcoital, intermenstrual bleeding or abnormal vaginal discharge.
She uses condoms for contraception and tried a COCP when she presented to a GP with painful periods at age 17 but stopped because it caused low mood. She currently manages the pain with heat packs and ibuprofen PRN.
|
Further history
- G0P0
- No previous history of STI
- CST – to commence age 25
- History of depression age 16–18
- No regular medications
- No family history of endometriosis
- Non-smoker
- Four to five standard drinks per week
- Eats pre-prepared dinner meals 3–4 times a week
- Yoga 1 h once or twice a week, gym 1 h twice a week
|
Physical examination and bedside tests
- Blood pressure 111/69, pulse 69 bpm, temperature 36.8°C
- Height 168 cm, weight 58 kg, body mass index 21 kg/m2
- Urinary human chorionic gonadotropin negative
- Urinalysis: leukocytes +1, nitrites negative, blood negative
- Abdominal exam: soft, generalised tenderness across pelvic region, no masses
- Pelvic and speculum exam: normal sized, mobile, anteverted uterus; no cervical motion or adnexal tenderness, no nodules or masses palpated; tender rectovaginal septum and tense obturator internus muscles bilaterally; no vaginal endometriotic lesions visualised, normal appearance of cervix
|
Georgie’s GP arranges the following investigations:
- Vaginal and urine MCS
- Chlamydia and gonorrhoea PCR
- Full blood count, C-reactive protein
- Pelvic ultrasonography (a dedicated gynaecological ultrasound service is available in Georgie’s suburb)
They discuss the possibility of endometriosis, and her GP recommends commencing empirical management with hormonal therapy, such as COCP or Mirena IUD. Georgie is unsure about recommencing hormonal therapy. Her GP provides resources for further reading and plans further discussion on review of her investigations. The GP suggests commencing a symptom diary. |
|
Georgie returns to review her results. Her blood tests, vaginal and urine MCS and STI screen are normal. Pelvic ultrasonography reports no endometriotic lesions visualised, sliding sign present, generalised probe tenderness, particularly in the rectovaginal septum. Normal appearance of uterus and ovaries; small amount of free-fluid in the pouch of Douglas.
Her GP explains that generalised tenderness on examinations and her clinical symptoms are suggestive of endometriosis. A presumed diagnosis is made and the GP recommends a chronic disease management plan and referral for pelvic floor physiotherapy. Georgie has decided to trial the COCP. She has no contraindications.
|
|
Georgie attends 3 months later, reporting a significant reduction in her mood, which her boyfriend and family have also noticed. She’s starting to fall behind in her studies, and although there has been a small reduction in her period pain, her overall quality of life has been reduced. Pelvic floor physiotherapy seems to be reducing her dyspareunia; however, she is now experiencing low libido. She has no suicidal ideation.
Georgie and her GP decide to stop the COCP, refer her to a gynaecologist with a special interest in endometriosis and review her mood in 4 weeks.
|
|
Georgie’s mood improves once the COCP has been ceased, and she has an appointment with the gynaecologist in 8 weeks’ time. She has joined a pelvic pain support group, which she is finding very beneficial. Georgie undergoes a laparoscopy, which diagnoses stage 3 endometriosis. A Mirena IUD is inserted at the time of surgery.
Six months following her surgery, Georgie is amenorrhoeic with the Mirena in situ and is no longer experiencing pelvic pain or deep dyspareunia. Six-monthly review of her management is scheduled. She continues a regular pelvic floor stretching regime.
|
| COCP, combined oral contraceptive pill; CST, cervical screening test; GP, general practitioner; IUD, intrauterine device; MCS, microscopy, culture and sensitivity; PCR, polymerase chain reaction; STI, sexually transmitted infection. |
Clinical examination
The diagnostic accuracy of pelvic examination for the detection of endometriosis is low. Therefore, a normal exam does not exclude a diagnosis. A pelvic exam is an important initial assessment of undifferentiated pelvic pain, and all three guidelines agree that it should be offered where appropriate. Findings on exam that might indicate endometriosis include palpable tethering of pelvic organs (eg uterine immobility), tender pelvic nodularity and visible vaginal lesions, particularly in the posterior fornix.16 It is reasonable to have a discussion with adolescents and their caregivers regarding a pelvic exam. Where it is not deemed appropriate, an abdominal exam can be offered to exclude pelvic masses.
Imaging
Transvaginal ultrasound scans (USS) and magnetic resonance imaging (MRI) are increasingly able to detect endometriomas and DIE. High-quality transvaginal USS (TVUSS) is as sensitive and specific as MRI in the detection of endometriomas and deep endometriosis. Laparoscopy remains superior for the detection of superficial endometriosis. The RANZCOG2 and NICE3 guidelines suggest TVUSS as the primary imaging modality; the ESHRE1 guideline also includes MRI. In Australia, MRI is only subsidised by Medicare for the purpose of surgical planning when DIE involving the bowel, bladder and ureter is suspected. The appropriateness of TVUSS needs to be considered when caring for adolescents. If it is not deemed appropriate, the ESHRE1 guideline states that MRI or transabdominal USS can be considered. The RANZCOG2 and NICE3 guidelines recommend transabdominal USS; however, the diagnostic accuracy is low.
Diagnostic laparoscopy
Diagnostic laparoscopy with histological confirmation has traditionally been considered the gold standard for the diagnosis of endometriosis. Although, increasingly, a clinical diagnosis of endometriosis is possible, all three guidelines emphasise that a normal exam and imaging cannot exclude endometriosis, and people with suspected endometriosis should be referred to a gynaecologist for further consideration of laparoscopy.1–3 Studies on endometriosis-confirmed laparoscopically in adolescents demonstrated that all stages of endometriosis can be present.17 The RANZCOG and NICE guidelines recommend considering specialised ultrasound or MRI to assess the extent of suspected deep endometriosis involving the bladder, bowel or ureter prior to surgery. Biopsy should be taken at the time of laparoscopy. However, a negative histological result does not rule out endometriosis.1–3 Figure 1 demonstrates the diagnostic algorithm provided in the ESHRE guideline.
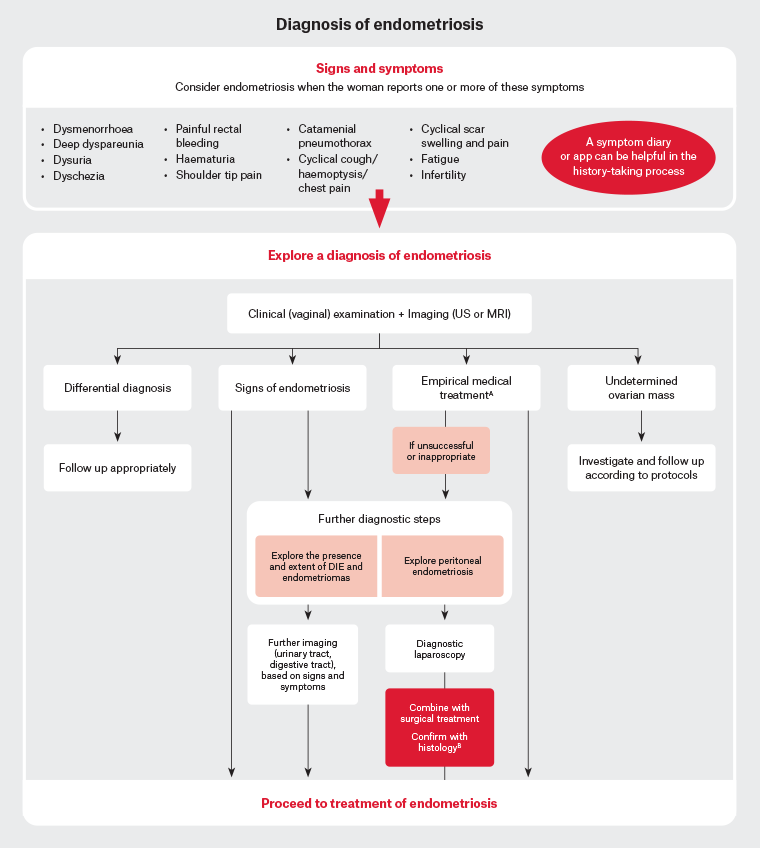
Figure 1. Diagnosis of endometriosis. Click here to enlarge
AEmpirical treatment = Combined hormonal contraceptive or progestogens. BBe aware that negative histology does not rule out endometriosis. The Royal Australian College of Obstetricians and Gynaecologists (RANZCOG)8 guideline recommends magnetic resonance imaging (MRI) for the purpose of surgical planning when deep infiltrating endometriosis (DIE) of the bowel, ureter or bladder is suspected as per current Medicare Benefits Schedule criteria.
Adapted from the European Society of Human Reproduction and Embryology (ESHRE) endometriosis guideline with permission from ESHRE.7
Biomarkers
There are no biomarkers with sufficient sensitivity or specificity to warrant use in the workup of endometriosis. All three guidelines state that biomarkers should not be used in the workup of endometriosis.1–3 Most recently, micro-RNAs involved in the genetic expression of processes crucial to endometriosis pathogenesis have shown some potential. However, validation in large, independent trials is required.18
Management of endometriosis‑associated pain
Pharmacological management
Analgesia
Paracetamol and non-steroidal anti-inflammatories either alone or in combination might be offered for the management of endometriosis-related pain.7–9 The evidence on their effect on endometriosis-related pain is limited to one small randomised controlled trial using naproxen, for which the results were inconclusive. The guidelines suggest a short trial of paracetamol and/or non-steroidal anti-inflammatories. If they do not provide adequate pain relief, other management options should be considered and the patient should be referred for further assessment.7–9
Neuromodulators
There are no studies evaluating the effectiveness of anti-neuropathic medications for endometriosis-related pain. The RANZCOG guideline suggests they might be used as a broader chronic pain management strategy following referral to a pain or condition-specific specialist.8
Hormone treatments
The use of hormonal treatments is based on evidence that endometriosis is a steroid-dependent condition.7 Treatments aim to reduce pain and induce atrophy of hormonally active endometriotic lesions by suppressing ovarian function. The most prescribed hormonal treatments include the combined oral contraceptive pill (COCP), progestogens (oral, depot, implant or intrauterine device [IUD]) and gonadotropin-releasing hormone (GnRH) agonists and antagonists.7 These hormonal therapies have been shown to lead to a clinically significant reduction in dysmenorrhoea and non-menstrual pelvic pain, without any demonstrating clear superiority.8 Patient preferences, priorities and tolerability should be considered when choosing whether to commence hormonal treatment and the type of therapy to commence.7–9 Table 3 provides a summary of hormonal treatments. All three guidelines recommend offering the COCP or progestogen to people with suspected, confirmed or recurrent endometriosis. The ESHRE guideline recommends that GnRH agonists can be considered as a second-line therapy, whereas the RANZCOG guideline recommends their use only as an adjunct to surgery for DIE involving the bowel, bladder or ureters. The use of GnRH agonists requires specialist advice. Hormonal therapies should be avoided in those wishing to conceive.
| Table 3. Hormonal therapies |
| |
Hormone |
Delivery method |
Contraceptive |
Administration |
Side effects |
Contraindications |
| Combined oral contraceptive pill |
No evidence of superiority of any one preparation |
Oral, vaginal ring or transdermal patch |
Yes |
- Cyclical or continuous
- Continuous further reduces dysmenorrhoea
|
Nausea, headaches, weight gain, breakthrough bleeding, mood disturbance, VTE |
History of VTE, IHD, migraine with aura, ≥35 years smoker ≥15/day, breast cancer, severe liver disease |
| Progestogens |
|
|
|
|
Breast tenderness, irregular bleeding, amenorrhoea, acne, mood disturbance |
Breast cancer, severe liver disease, unexplained vaginal bleeding |
| |
Levonorgestrel 52 mg (Mirena) |
Intrauterine device |
Yes |
Replace 5 yearly |
PID, expulsion, perforation, post-insertion cramping, ovarian cyst formation |
PID, active STI, cervical or endometrial cancer |
| |
Medroxyprogesterone 150 mg (Depo-Provera) |
Intramuscular injection |
Yes |
Injection 3 monthly |
Injection site pain, delayed return to fertility, reduced BMDA |
Refer above |
| |
Etonogestrel 68 mg (Implanon) |
Subdermal implant |
Yes |
Replace 3 yearly |
Implant site pain, infection, migration |
| |
Medroxyprogesterone |
Oral |
No |
10 mg 3 times daily |
Refer above |
| |
Dienogest |
Oral |
No |
2 mg once daily |
| |
Norethisterone |
Oral |
Yes |
5–10 mg once daily |
| |
DrospirenoneB |
Oral |
Yes |
4 mg once daily |
| Gonadotropin-releasing agonists (under specialist supervision) |
|
|
|
Maximum 6 months |
|
|
| Goserelin |
Subcutaneous |
No |
3.6 mg injection 4 weekly |
Hot flushes, vaginal dryness, sexual dysfunction, amenorrhoea, reduced BMD |
Breastfeeding, pregnancy, unexplained vaginal bleeding, osteoporosis |
| |
Nafarelin |
Intranasal |
No |
200 µg twice daily |
ABone mineral density (BMD) usually recovers within 2 years of discontinuation.
BCurrently off-label for endometriosis use.
BMD, bone mineral density; IHD, ischaemic heart disease; PID, pelvic inflammatory disease; STI, sexually transmitted infection; VTE, venous thromboembolism. |
Non-pharmacological management
A focus on education and awareness of endometriosis is a key priority of the Australian National Action Plan for Endometriosis. Education allows patients to be empowered, active decision-makers in their healthcare. A list of educational tools is provided in Table 4.
Sixty to seventy per cent of people utilise some form of non-medical management, including acupuncture, Chinese herbal medicine, physiotherapy, exercise and nutritional strategies.19 Engaging in non-medical management strategies can help people to regain a sense of control over chronic conditions. The recommendation for their use, however, is limited by the lack of high-quality studies evaluating their effectiveness and safety. Hence, all three guidelines advise that no recommendation can be made for any specific non-medical intervention.1–3
People with evidence of concurrent pelvic floor dysfunction might benefit from pelvic floor physiotherapy.20 It is important that clinicians appreciate the psychological effects of living with chronic pain and/or infertility, including anxiety and depression, where the evidence of the benefits of psychological strategies such as cognitive behavioural therapy and mindfulness is robust.7
Surgical management
Surgical management of endometriosis is generally performed via laparoscopy. Surgery aims to remove endometrial deposits and correct distortions to anatomy caused by adhesions and scar tissue. Surgery has been shown to result in an overall improvement in most health-related quality of life (QoL) domains.21
Major surgical complications are higher when DIE is present, particularly involving the bowel.22 The three guidelines recommend that women with DIE be referred to a centre of expertise or a multidisciplinary team with expertise in this area.7–9 Cystectomy of endometriomas is recommended by all three guidelines as it reduces the risk of recurrence and pain and increases spontaneous pregnancy rates compared to ablation.23 However, this needs to be carefully weighed up against its effect on ovarian reserve, especially in young women not immediately seeking fertility.7
Secondary recurrence
It is important to counsel women that surgery is not a cure for endometriosis. Surgery aims to remove any visible lesions. However, relapse of symptoms occurs in 40–45% of women. Up to 30% of women are readmitted for surgery within five years of their first surgery.24 Postoperative hormonal suppression via levonorgestrel-IUD (LNG-IUD) or continuous COCP might have some benefit for the secondary prevention of pain and disease recurrence.25,26 All three guidelines recommend considering hormonal treatment following laparoscopy for endometriosis.
Hysterectomy
Hysterectomy (with or without removal of the ovaries) might be considered if women have completed their family and where medical and surgical interventions have been ineffective or where there is concurrent heavy menstrual bleeding and/or adenomyosis.1–3 Removal of all visible lesions should be performed at the same time. When considering bilateral oophorectomy, the increased risk of diminished bone density, dementia and cardiovascular disease should be discussed. Women should be counselled that pain symptoms might persist or recur due to persisting or recurring endometriosis and/or central sensitisation.7,8
Management of endometriosis-associated infertility
Endometriosis is present in up to 50% of women experiencing infertility and subfertility.2 Management might involve expectant, surgical and/or assisted methods of reproduction (ART). In women with endometriosis experiencing infertility or subfertility, an interdisciplinary approach with shared decision making should be undertaken. This should include a specialist with an interest in endometriosis-related infertility to appropriately counsel patients regarding treatment options.
Surgical management
The three guidelines7–9 agree that offering surgical management of rASRM stage 1 and 2 endometriosis might improve fertility outcomes. The data for fertility outcomes in surgical management of rASRM stages 3 and 4 are less clear. The RANZCOG guideline8 recommends consultation with a fertility expert with experience in endometriosis when ovarian endometriomas are present, with the judicious use of ovarian surgery given its effect on ovarian reserve. All three guidelines agree that hormonal suppression should not be offered postoperatively, when fertility is a main priority.7–9
Assisted reproduction technology
The ESHRE guideline suggests intrauterine insemination (IUI) with ovarian stimulation might be performed in women with rASRM stage 1 and 2 endometriosis, as pregnancy rates are higher in this group than expectant management or IUI alone.7 In vitro fertilisation (IVF) is recommended if tubal function is compromised, there is male factor infertility or other treatments have failed. The ESHRE guideline reassures that ART is safe to use in women with endometriosis, given there is no difference in reoccurrence rates between women who have undergone ART and women who have not.27
Fertility preservation
The ESHRE guideline considers the increased risk of premature ovarian insufficiency and lower anti-mullerian hormone (AMH) levels in patients with severe endometriosis (particularly bilateral endometriomas), acknowledging the further effects of surgical treatment on ovarian reserve. Therefore, the ESHRE guideline recommends counselling on the pros and cons of fertility preservation in women with extensive ovarian endometriosis, while acknowledging that the true benefit remains unclear.7
Pregnancy
The ESHRE guideline acknowledges that endometriosis might increase the risk of first trimester miscarriage and ectopic pregnancy.1 Pregnancy has a variable effect on endometriosis lesions; they might regress or grow.28 People should not be advised to become pregnant for the primary purpose of treating endometriosis.1
Endometriosis in menopause
The ESHRE guideline reports that two to five per cent of people will experience endometriosis-related symptoms after menopause.29 Differential diagnoses such as malignancy need to be considered when postmenopausal people present with pelvic pain. Evidence regarding optimal treatment of menopausal symptoms in people with a history of endometriosis is limited to predominantly case reports. A systematic review by Gemmell et al30 found that people with a history of endometriosis might experience reactivation of endometriosis when treating menopausal symptoms with menopausal hormone therapy, as well as an increased risk of malignant transformation of the lesions. The latter was found particularly with oestrogen-only preparations. Although the RANZCOG and NICE guidelines do not address the issue of treatment of menopausal symptoms in people with a history of endometriosis, the ESHRE guideline recommends that ‘to avoid reactivation of lesions and potential increased risk of malignant transformation, combined oestrogen–progesterone formulations should be used, including in people who have had a hysterectomy’.1
Conclusion
Endometriosis is a common yet underdiagnosed condition that can cause significant morbidity from adolescence to beyond menopause. Negative examination and imaging findings cannot rule out endometriosis, and prompt diagnosis and treatment of people with suspected or confirmed endometriosis are recommended as delays in treatment might significantly affect quality of life and disease progression. Medical management of suspected or confirmed endometriosis can be commenced in the general practice setting. However, prompt referral to a gynaecologist is recommended where imaging findings suggest a higher stage of disease, symptoms are severe or medical management is ineffective or not tolerated. Recurrence rates of endometriosis following surgical excision are high, and postsurgical hormonal suppression should be considered. People experiencing endometriosis infertility or subfertility should be referred to a specialist with an interest in endometriosis-related infertility. General practitioners are well placed to provide the continuity of care necessary for successful multidisciplinary team management of this complex condition.
Key points
- People with suspected or confirmed endometriosis should be offered comprehensive and coordinated care from their clinical team as delays can affect quality of life and disease progression. People with endometriosis meet the criteria for a chronic disease management plan.
- Refer young people (aged 17 years and under) with suspected or confirmed endometriosis to a paediatric and adolescent gynaecologist with an interest in endometriosis or to a gynaecologist who is comfortable treating adolescents with possible endometriosis.
- Laparoscopy should no longer be considered the gold standard for diagnosis of endometriosis but should be considered if imaging is negative and/or empirical treatment is ineffective or inappropriate.1
- Consider prescribing LNG-IUD or COCP postoperatively for the secondary prevention of endometriosis-associated dysmenorrhoea.
- In women with endometriosis experiencing infertility or subfertility, an interdisciplinary approach with shared decision making should be undertaken. This should include a specialist with an interest in endometriosis-related infertility to appropriately counsel patients regarding treatment options.