Semaglutide (Ozempic) and dulaglutide (Trulicity) are glucagon-like peptide-1 (GLP-1) receptor agonists subsidised via the Australian Pharmaceutical Benefits Scheme (PBS) and Repatriation PBS (RPBS) for the treatment of type 2 diabetes (T2D).1 As well as improving glycaemic control, this class of medication has also been shown to have benefit in body weight reduction.2 Prior to the release of semaglutide, liraglutide (Saxenda), a daily-dosing GLP-1 agonist, was often used in Australia for the purposes of weight loss. However, due to its daily dosing regimen and cost (approximately $400 per month), once semaglutide became available in Australia, off-label use of semaglutide (administered once weekly and costing approximately $180 per month) became the agent of choice for consumers.3 More recently, Wegovy, a once-weekly formulated semaglutide solution administered at 2.4 mg/week, has also shown clinically relevant benefit in weight reduction when used in combination with lifestyle modification strategies.4 Nevertheless, although registered on the Australian Register of Therapeutic Goods, Wegovy is not yet available in Australia.5 For clarity, we use the generic name semaglutide henceforth in this research article to refer to Ozempic specifically.
In April 2022, the Therapeutic Goods Administration (TGA) reported a shortage of semaglutide in Australia in the setting of a worldwide shortage of semaglutide due to an unexpected increase in demand.5 In the preceding months, there was significant media coverage regarding the use of semaglutide for weight loss, and it is suspected the global shortage was at least partially contributed to by the off-label use of this agent for weight management.5 In response to the semaglutide shortage, The Royal Australian College of General Practitioners (RACGP) advised general practitioners to exercise care and consideration with respect to off-label prescribing of semaglutide so as to ensure prioritised treatment of patients with T2D.6,7 In June 2022, the TGA announced a subsequent shortage of dulaglutide.8
The impact of semaglutide and dulaglutide shortages on T2D treatment in Australia is yet to be elucidated. This study aimed to measure the impact of semaglutide and dulaglutide shortages on their use in T2D treatment by examining trends in the actual and predicted number of PBS/RPBS prescriptions supplied during 2021 and 2022.
Methods
This retrospective population-based study examined semaglutide and dulaglutide prescriptions supplied via the PBS and RPBS between January 2021 and September 2022 for the treatment of T2D in Australia.
Database
The PBS/RPBS schedule lists semaglutide (PBS/RPBS Item no. 12080T and 12075M) and dulaglutide (Item no. 11364D) for T2D treatment,
9 allowing Australian citizens, permanent residents and veterans subsidised access to these medications. There are specific restrictions when prescribing semaglutide and dulaglutide via the PBS/RPBS (Table 1), including their use in combination with metformin, a sulfonylurea and/or insulin (and not other oral hypoglycaemic agents), and that the patient has a recorded HbA1c >7% or a consistently demonstrated blood glucose level >10 mmol/L.
9 Data reports recording the number of prescriptions supplied for specific PBS/RPBS item numbers are made publicly available by the Commonwealth of Australia.
10 Using these reports, the number of semaglutide (Item no. 12080T and 12075M) and dulaglutide (Item no. 11364D) prescriptions supplied in Australia each month between January 2021 and September 2022 via the PBS/RPBS were analysed retrospectively.
| Table 1. Specific restrictions for the supply of semaglutide (Ozempic) and dulaglutide (Trulicity) via the Pharmaceutical Benefits Scheme (PBS)/Repatriation PBS9 |
| (i) Must be used in combination with other T2D medications |
Metformin, or
Sulfonylurea,A and/or
Insulin
(Or patient must have a contraindication or be unable to tolerate metformin and/or sulfonylurea) |
| AND |
| (ii) Must not be used in combination with other T2D medications |
DPP4 inhibitor, or
Thiazolidinedione, and/or
SGLT2 inhibitor |
| AND |
| (iii) Patient must have HbA1c >7% or BGL >10 mmol/L |
HbA1c >7% or BGL >10 mmol/L in more than 20% of tests over a 2-week period prior to initiating treatment, despite treatment with metformin, sulfonylurea and/or insulin |
AOzempic might be prescribed in combination with a sulfonylurea, without metformin. However, Trulicity cannot be prescribed in combination with a sulfonylurea alone; treatment must also be in combination with metformin (unless the patient has a contraindication to or is unable to tolerate metformin).
BGL, blood glucose level; DPP4, dipeptidyl peptidase 4; SLGT2, sodium–glucose cotransporter 2; T2D, type 2 diabetes. |
Statistical analysis
Changes in the number of PBS/RPBS semaglutide and dulaglutide prescriptions supplied in each calendar month with time between January 2021 and September 2022 were analysed. The relative change in the number of prescriptions supplied month to month was calculated as a percentage. In addition, Holt–Winters forecast models were used to predict the number of PBS/RPBS semaglutide and dulaglutide prescriptions supplied each month between February and September 2022. Predicted prescription numbers were compared with the actual number of PBS/RPBS semaglutide and dulaglutide prescriptions supplied. The relative difference between the actual and predicted number of prescriptions supplied, and the 95% confidence interval (CI), was calculated as a percentage.
Holt–Winters forecast modelling is a commonly used analytical technique that uses previous observations in a time series to predict future observations. Holt–Winters models are used for forecasting data with changing trends and seasonal correlation, using triple exponential smoothing to account for three parameters: mean values (level), trend over time and seasonality.11 The weighted mean of past observations are used in the prediction, with weights decreasing exponentially as observations become older. When devising forecasting models, it is suggested that there are at least more observations than parameters.12 Holt–Winters models have been used previously to forecast monthly PBS prescriptions, including in a recent analysis investigating the impact of COVID-19 on medication dispensing in Australia.13 In the present study, PBS/RPBS semaglutide and dulaglutide prescription data between January 2021 and January 2022 were used to predict the number of semaglutide and dulaglutide prescriptions expected between February and September 2022. Given the semaglutide and dulaglutide shortages were reported in April and June 2022, respectively, comparisons were made for actual and predicted semaglutide prescriptions between April and July 2022, as well as actual and predicted dulaglutide prescriptions between June and July 2022. The relative difference between the actual and predicted number of semaglutide and dulaglutide prescriptions was calculated as a percentage. Semaglutide was listed on the PBS on 1 July 2020.14 Because exponential smoothing techniques are sensitive to outlier values,11 six months was given for prescription numbers to stabilise (ie January 2021 onwards data were used in the forecast models); the intention was to minimise error in prediction, particularly overestimation.
Data analyses were performed using Stata/MP 17.0 Data Analysis and Statistical Software (StataCorp, College Station, TX, USA). Ethics approval was not sought for this analysis given the data are publicly available.
Results
Between January 2021 and January 2022, the actual number of PBS/RPBS semaglutide prescriptions supplied in Australia per month increased 5.3-fold, from 15,085 to 80,357 prescriptions, respectively (Figure 1). The actual number of PBS semaglutide prescriptions supplied peaked at 96,504 in March 2022, before falling overall by 17% by September 2022, when only 80,352 prescriptions were supplied.
In comparison, the actual number of PBS/RPBS dulaglutide prescriptions supplied in Australia tended to increase between January 2021 and January 2022, rising 36% from 45,289 to 61,744 prescriptions, respectively. The number of actual PBS/RPBS dulaglutide prescriptions supplied in January 2022 was similar to the number supplied in March 2022 (58,712 prescriptions). Thereafter, dulaglutide prescriptions supplied increased each month between April 2022 (55,869 prescriptions) and peak levels in July 2022 (85,378 prescriptions), rising overall by 53%. This was then followed by a 17% fall in dulaglutide prescriptions supplied between August (71,893 prescriptions) and September 2022 (59,767 prescriptions).
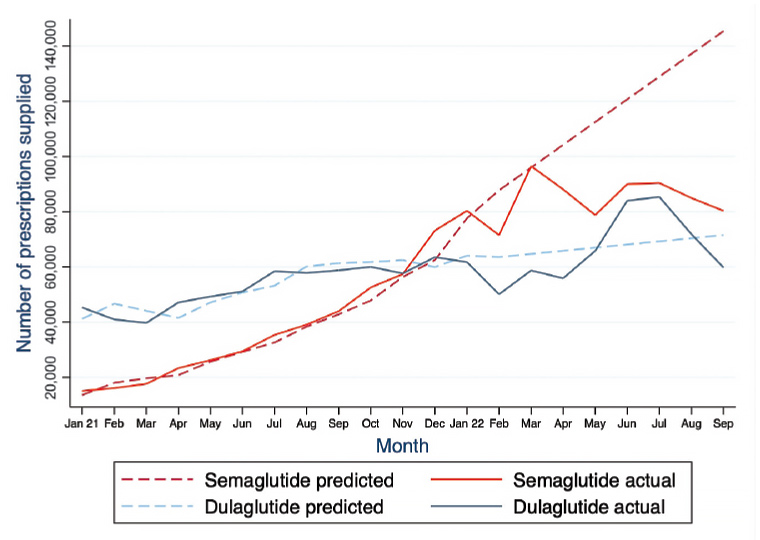
Figure 1. Number of predicted and actual semaglutide (Ozempic) and dulaglutide (Trulicity) prescriptions supplied for the treatment of type 2 diabetes in Australia via the Pharmaceutical Benefits Scheme (PBS) and Repatriation PBS in each calendar month between January 2021 and September 2022.
When analysing these data using Holt–Winters forecast modelling, and comparing actual and predicted prescription numbers, 119,069 fewer semaglutide prescriptions were actually supplied than predicted between April and July 2022 (–26%; 95% CI: –30% to –21%; Figure 1). In contrast, 31,953 more dulaglutide prescriptions were actually supplied than predicted between June and July 2022 (+19%; 95% CI: +10% to +28%).
Discussion
In this retrospective population-based study, actual and predicted semaglutide and dulaglutide prescriptions supplied for T2D treatment in Australia via the PBS and RPBS between January 2021 and September 2022 were examined. Prescription supply patterns varied during the study period, with changes in the actual number of prescriptions supplied coinciding with TGA announcements of semaglutide and dulaglutide supply shortages in 2022.
Prior to the TGA announcement of a semaglutide shortage in April 2022, the actual number of semaglutide prescriptions supplied in Australia increased each month and overall by 5.3-fold in the 12-month period between January 2021 and January 2022. Then, between March and September 2022, the actual number of semaglutide prescriptions decreased by 17%. The prediction model results of the study suggest that the decrease in semaglutide prescriptions supplied at the time of the shortage might be more, with 119,069 fewer prescriptions (a 26% relative decrease) of semaglutide actually supplied than predicted between April and July 2022. Coinciding with the decrease in semaglutide prescriptions between April and July 2022, actual dulaglutide prescriptions increased by 53% between these same months. Prior to this, actual dulaglutide prescriptions had comparatively increased overall by 36% during the 13-month period between January 2021 and January 2022. The prediction model findings similarly suggest that the increase in dulaglutide prescriptions supplied between June and July 2022 might be more, with 31,953 more prescriptions (a 19% relative increase) of dulaglutide actually supplied than predicted. Following the TGA’s announcement of a dulaglutide shortage in June 2022, actual dulaglutide prescriptions fell 17% between August and September 2022.
Although this study does not include prescriptions supplied privately in Australia during the study period, changes in the number of semaglutide and dulaglutide prescriptions supplied via the PBS/RPBS for T2D treatment in 2022 correlated with the timing of their respective shortages announced by the TGA. The prediction model results of this study highlighted that the decrease in semaglutide prescriptions might be more exaggerated than the fall seen in the actual number of semaglutide prescriptions between April and July 2022 following the April TGA announcement of a semaglutide shortage.5 A plausible explanation for the increase in actual dulaglutide prescriptions during the subsequent months, 19% above the number predicted between June and July 2022, might be clinicians prescribing dulaglutide as an alternative T2D therapy to semaglutide. This was likely until dulaglutide itself became difficult to access in August 2022 onwards, as possibly evidenced by the 17% decrease in actual dulaglutide prescriptions supplied between August and September 2022.
Thus far, semaglutide and dulaglutide shortages have continued in 2023.5,8 Novo Nordisk, the pharmaceutical company responsible for manufacturing and supplying semaglutide, informed the TGA that limited semaglutide stock is commencing distribution in Australia and will be available at some community pharmacies; however, initial supplies will not be enough to meet demand from all patients with a prescription for T2D.5,15 Eli Lilly, the pharmaceutical company responsible for manufacturing and supplying dulaglutide, informed the TGA that there will be limited availability of dulaglutide until the end of December 2023.8 At present, it is unclear when both agents’ supply in Australia will return in full to meet demand.
For general practitioners, the study findings reaffirm the RACGP’s and TGA’s recent statements advising prescribers to prioritise the use of semaglutide for patients with T2D who are either current users or are previous users and for whom other glucose-lowering medications, including sulfonylureas, dipeptidyl peptidase 4 inhibitors and sodium–glucose cotransporter 2 inhibitors (Table 1), are unsuitable due to refractory or worsening glycaemic control, intolerance or contraindication.
15,16 Where possible, clinicians should also avoid commencing new patients on semaglutide until supply stabilises. In patients with persistent hyperglycaemia, in particular those without weight concerns, consideration could be given to commencing insulin therapy meanwhile. It is recommended that clinicians and pharmacists strongly prioritise the use of these medications for their appropriate T2D PBS/RPBS indication in the immediate future (Table 1), until abundant supply can be guaranteed nationwide.
In addition, in response to the semaglutide and dulaglutide shortages, the TGA is endeavouring to ensure the fair distribution of these medications in the future.5,8 At its March 2023 meeting, the Pharmaceutical Benefits Advisory Committee (PBAC) also recommended that the PBS authority prescription of semaglutide and dulaglutide for patients being initiated on the agents be changed from STREAMLINED to written or telephone approval.17 Furthermore, formal guidelines regarding the use of alternative therapies should be widely advertised to clinicians, and monthly stock availability updates should be sent to all members of the medical college to facilitate the appropriate use of these agents among patients most at need.
Finally, future studies evaluating the safety and cost-effectiveness of GLP-1 agonists, such as semaglutide formulated as Wegovy, for their weight loss benefit are needed in Australia. Such information would be useful to the PBAC. Obesity remains an issue of public health importance in Australia, and ensuring the availability of a safe and affordable agent to assist with weight reduction under the PBS wherever possible would likely be of benefit to the wider community. At a broader level, the shortage of semaglutide and dulaglutide during the COVID-19 pandemic highlights the importance for health policymakers to proactively consider methods to protect the supply chain in the future, particularly for essential medications available via the PBS.
The strengths of this study are its population-based design, with PBS/RPBS prescription supply data highly representative of medication use by the Australian population. Furthermore, the use of a prediction model, which allowed comparison of actual and predicted PBS/RPBS semaglutide and dulaglutide prescription numbers supplied, increases confidence that trends observed in actual prescriptions supplied over time are not artefactual.
There are also limitations to this study. The ecological design of this analysis means causation cannot be implied; the study results only indicate changing PBS/RPBS semaglutide and dulaglutide prescription patterns at the time of the TGA medication shortage announcements. Moreover, because semaglutide was only listed on the PBS Schedule in July 2020, with six months lag time allowed for prescription numbers to stabilise to minimise prediction error, limited data were available to include in the forecast models and to account for mean values, trends with time and seasonality, and the potential impact of COVID-19 on prescription numbers. It is also possible domestic restrictions during the COVID-19 pandemic might have contributed, in part, although likely to a lesser extent, to the shortage in Australia. In addition to the lack of data relating to semaglutide and dulaglutide prescriptions supplied privately, and not via the PBS/RPBS, other confounders impacting the prescription of these agents, such as poor tolerance and side effects, have not been included in the study analysis.
Conclusion
This study demonstrated changes in the actual number of semaglutide and dulaglutide prescriptions supplied for T2D treatment between January and September 2022, with reductions in the number of semaglutide and dulaglutide prescriptions supplied coinciding with the TGA announcements of shortages for semaglutide and dulaglutide in April and June 2022, respectively. The prediction model results further highlight the extent of the change in actual prescriptions supplied at the time of the shortages, with the decrease in semaglutide prescriptions likely to be more than the fall seen in actual semaglutide prescription numbers in the months following the TGA semaglutide shortage announcement.